
The Recombinant Protein Handbook Protein Amplification and Simple Purification Amersham 18-1142-75 Biosciences Edition AB
18-1142-75 Edition AB Protein Amplification and Simple Purification The Recombinant Protein Handbook

The Recombinant Protein Handbook Protein Amplification and Simple Purification
1 The Recombinant Protein Handbook Protein Amplification and Simple Purification

Contents Introduction 5 Symbols and abbreviations CHAPTER 1 6 Choice of ost for Choice of vec or Vectors for fusion proteins Choice of fusion tag. CHAPTER 2. shootine protein amplification CHAPTER 3. GST fusion proteins. 13 Amplification DeconoGsTfsonpoeins 12 Purification and detection troubleshooting. 203 Thrombin cleavage and purification 3 FactorXaceareandpu0tcaic ie protease CHAPTER 4. .41 (His).fusion proteins 41 Amplification detection troubes Tag removal by enzymatic cleavage CHAPTER 5. 59 Handling inclusion bodie RetoldingotsolubilizedTecoambinantpotei5 CHAPTER 6. .63 Harvesting and extraction of recombinant proteins .63 CHAPTER 7. 67 Buffer exchange and desalting of recombinant proteins CHAPTER 8. Simple purification of other recombinant proteins. .71 Ready to use affinity purification os 1
2 Contents Introduction . 5 Symbols and abbreviations . 5 CHAPTER 1 . 6 Choice of host for protein amplification . 6 Choice of vectors . 7 Vectors for non-fusion proteins . 7 Vectors for fusion proteins . 8 Choice of fusion tag . 8 CHAPTER 2 . 9 Protein amplification . 9 Sample extraction . 9 Troubleshooting protein amplification . 9 CHAPTER 3 . 13 GST fusion proteins . 13 Amplification . 13 Purification . 14 Detection of GST fusion proteins . 21 Purification and detection troubleshooting . 28 Tag removal by enzymatic cleavage . 30 PreScission Protease cleavage and purification . 31 Thrombin cleavage and purification . 35 Factor Xa cleavage and purification . 37 Removal of thrombin, Factor Xa or other serine proteases . 39 CHAPTER 4 . 41 (His)6 fusion proteins . 41 Amplification . 41 Purification . 41 Detection of (His)6 fusion proteins . 53 Purification and detection troubleshooting . 56 Tag removal by enzymatic cleavage . 58 CHAPTER 5 . 59 Handling inclusion bodies . 59 Solubilization of inclusion bodies . 59 Refolding of solubilized recombinant proteins . 60 CHAPTER 6 . 63 Harvesting and extraction of recombinant proteins . 63 CHAPTER 7 . 67 Buffer exchange and desalting of recombinant proteins . 67 CHAPTER 8 . 71 Simple purification of other recombinant proteins . 71 Ready to use affinity purification columns . 71 Making a specific purification column . 73 Purification . 75

CHAPTER 9. 77 Multi-step purification of recombinant proteins(fusion and non-fusion). Appendix 1. 86 Map of the GST fusion vectors showing reading frames and main features Glutathione S-transferase (GST) Appendix 2. .88 Amino acids table Appendix 3. 90 Protein conversion data Appendix 4. 90 Centrifuges,rotors and carriers for use with MicroPlex 24 % Appendix 5. .91 tics Characteristics,cleaning and storage of Chelating Sepharose Appendix 6. 93 Column packing and preparation Appendix 7. 95 Converting from linear flow (cm/hour)to volumetric flow rates (ml/min)and vice versa. Appendix 8. 96 Selection of purification equipment % Appendix 9. 97 s and stan onditions for purification techniques o Exchange 97 Hydrophobic n tion Chromatography (HIC) 101 Additional reading and reference material ,104 Orde ring infor 105
3 CHAPTER 9 . 77 Multi-step purification of recombinant proteins (fusion and non-fusion) . 77 Selection and combination of purification techniques . 78 Appendix 1 . 86 Map of the GST fusion vectors showing reading frames and main features . 86 Glutathione S-transferase (GST) . 87 Appendix 2 . 88 Amino acids table. 88 Appendix 3 . 90 Protein conversion data . 90 Appendix 4. . 90 Centrifuges, rotors and carriers for use with MicroPlex 24 . 90 Appendix 5 . 91 Characteristics, cleaning and storage of Glutathione Sepharose . 91 Characteristics, cleaning and storage of Chelating Sepharose . 92 Appendix 6 . 93 Column packing and preparation . 93 Appendix 7 . 95 Converting from linear flow (cm/hour) to volumetric flow rates (ml/min) and vice versa . 95 Appendix 8 . 96 Selection of purification equipment . 96 Appendix 9 . 97 Principles and standard conditions for purification techniques . 97 Affinity Chromatography (AC) . 97 Ion Exchange (IEX) . 97 Hydrophobic Interaction Chromatography (HIC) . 99 Gel Filtration (GF) Chromatography . 100 Reversed Phase Chromatography (RPC) . 101 Additional reading and reference material . 104 Ordering information . 105
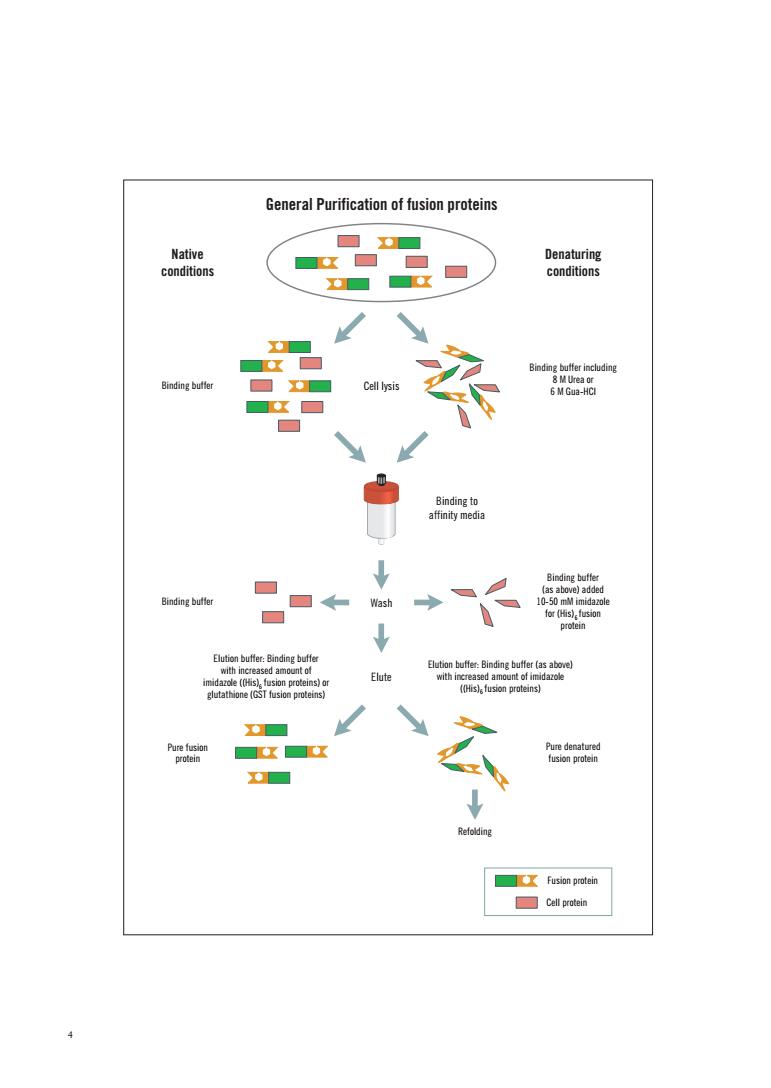
General Purification of fusion proteins Fesion protein
4 Native conditions Binding buffer Binding buffer Denaturing conditions Cell lysis Binding buffer including 8 M Urea or 6 M Gua-HCl Binding to affinity media Wash Elute Pure denatured fusion protein Pure fusion protein Refolding Binding buffer (as above) added 10-50 mM imidazole for (His) fusion protein 6 Elution buffer: Binding buffer (as above) with increased amount of imidazole ((His) fusion proteins) 6 Elution buffer: Binding buffer with increased amount of imidazole ((His) fusion proteins) or glutathione (GST fusion proteins) 6 General Purification of fusion proteins Fusion protein Cell protein

Introduction This handbook is intended for the general reader interested in the amplification and purification of recombinant proteins and for everyday use at the laboratory bench. The use of recombinant proteins has increased greatly in recent years,as has the wealth of techniques and products used for their amplification and purification.The advantages of using a fusion protein to facilitate purification and detection of the recombinant proteins are now widely recognised.This handbook introduces the reader to the initial considerations to be made when deciding upon host,vector and use of a fusion or non-fusion protein and covers general guidelines for successful protein amplification.General advice is also given on harvesting and extraction,handling of inclusion bodies,tag removal and removal of unwanted salts and small molecules. The more that is known about the characteristics of a protein,the more easily it can be isolated and purified.Consequently,fusion proteins are simple and convenient to work with and,for many applications,a single purification step,using a commercially available affinity chromatography column,is sufficient.This is clearly demonstrated in the specific chapters on the amplification,purification and detection of the two most common fusion proteins(GST and (His)tagged proteins)which include simple practical protocols for use in the laboratory.The handbook also gives suggestions for the successful purification of other fusion proteins by a single affinity chromatography step. required,a multi-step purification will be necessary.This can al forward task by following a Three Phase Purification Strategy reviewed in the final chapter. Symbols and abbreviations this symbo gives general advice that can improve procedures and provides recommendations for action under specific situations. 业 this symbol denotes advice that should be arded as mandatory and gives a warning when this symbol gives troubleshooting advice to help analyse and resolve any difficulties which may occur. reagents and equipment required experimental protocol. PBS phosphate buffered saline
5 Introduction This handbook is intended for the general reader interested in the amplification and purification of recombinant proteins and for everyday use at the laboratory bench. The use of recombinant proteins has increased greatly in recent years, as has the wealth of techniques and products used for their amplification and purification. The advantages of using a fusion protein to facilitate purification and detection of the recombinant proteins are now widely recognised. This handbook introduces the reader to the initial considerations to be made when deciding upon host, vector and use of a fusion or non-fusion protein and covers general guidelines for successful protein amplification. General advice is also given on harvesting and extraction, handling of inclusion bodies, tag removal and removal of unwanted salts and small molecules. The more that is known about the characteristics of a protein, the more easily it can be isolated and purified. Consequently, fusion proteins are simple and convenient to work with and, for many applications, a single purification step, using a commercially available affinity chromatography column, is sufficient. This is clearly demonstrated in the specific chapters on the amplification, purification and detection of the two most common fusion proteins (GST and (His)6 tagged proteins) which include simple practical protocols for use in the laboratory. The handbook also gives suggestions for the successful purification of other fusion proteins by a single affinity chromatography step. In situations where no fusion system is available, or when a higher degree of purity is required, a multi-step purification will be necessary. This can also become a straightforward task by following a Three Phase Purification Strategy reviewed in the final chapter. Symbols and abbreviations this symbol gives general advice that can improve procedures and provides recommendations for action under specific situations. this symbol denotes advice that should be regarded as mandatory and gives a warning when special care should be taken in a procedure. this symbol gives troubleshooting advice to help analyse and resolve any difficulties which may occur. reagents and equipment required. experimental protocol. PBS phosphate buffered saline
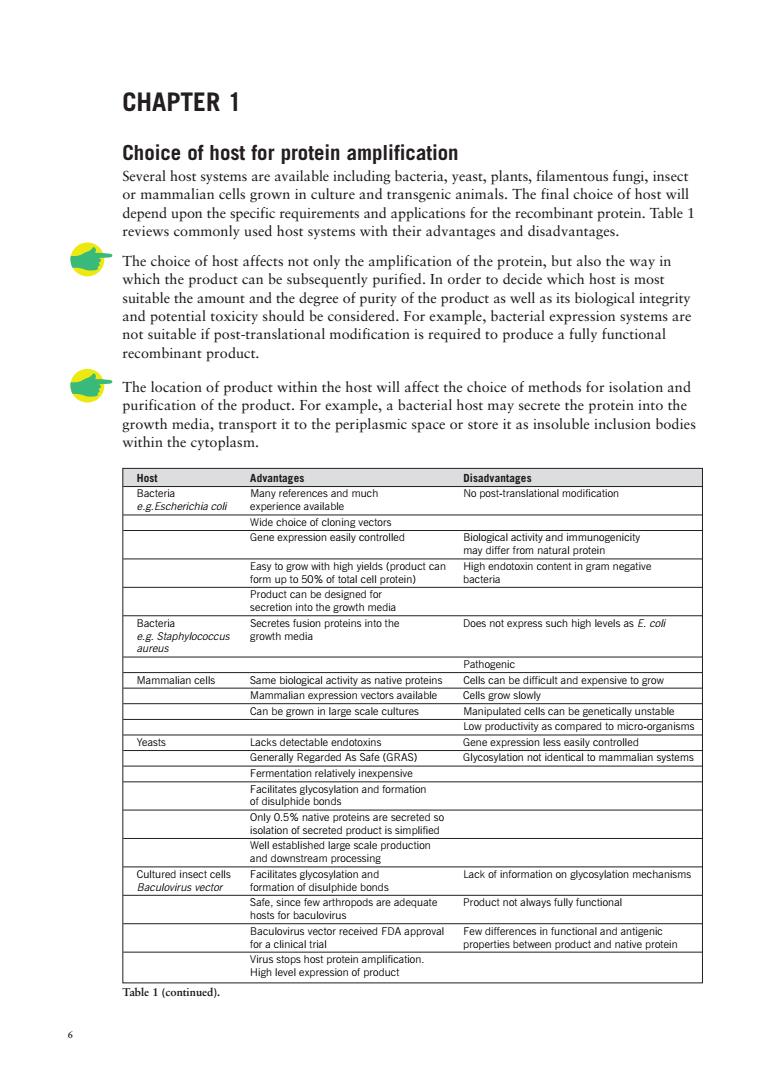
CHAPTER 1 Choice of host for protein amplification Several host systems are available including bacteria,yeast,plants,filamentous fungi,insect or mammalian cells grown in culture and transgenic animals.The final choice of host will depend upon the specific requirements and applications for the recombinant protein.Table 1 reviews commonly used host systems with their advantages and disadvantages. The choice of host affects not only the amplification of the protein,but also the way in which the product can be subsequently purified.In order to decide which host is most suitable the amount and the degree of purity of the product as well as its biological integrity and potential toxicity should be considered.For example,bacterial expression systems are not suitable if post-translational modification is required to produce a fully functional recombinant product. inclusion bodies Hos and much Not-ranstnmodfcti Mide choice of cloning vectors Gene expression easily controlled Easy to gro Bacteria Does not express such high levels as E.colf Mammalian cells ammalian expression vectors available Cells grow slowly an be grown in large scale cultures lls can be gen ally unstable d to Yeasts Lacks dete ene expresson less easily an systems on and formation tion and Lack of information on glycosylation mechanisms rth for bac Product not always fully functional egmC5teSoreceedFDAappova Table 1(continud)
6 CHAPTER 1 Choice of host for protein amplification Several host systems are available including bacteria, yeast, plants, filamentous fungi, insect or mammalian cells grown in culture and transgenic animals. The final choice of host will depend upon the specific requirements and applications for the recombinant protein. Table 1 reviews commonly used host systems with their advantages and disadvantages. The choice of host affects not only the amplification of the protein, but also the way in which the product can be subsequently purified. In order to decide which host is most suitable the amount and the degree of purity of the product as well as its biological integrity and potential toxicity should be considered. For example, bacterial expression systems are not suitable if post-translational modification is required to produce a fully functional recombinant product. The location of product within the host will affect the choice of methods for isolation and purification of the product. For example, a bacterial host may secrete the protein into the growth media, transport it to the periplasmic space or store it as insoluble inclusion bodies within the cytoplasm. Host Advantages Disadvantages Bacteria Many references and much No post-translational modification e.g.Escherichia coli experience available Wide choice of cloning vectors Gene expression easily controlled Biological activity and immunogenicity may differ from natural protein Easy to grow with high yields (product can High endotoxin content in gram negative form up to 50% of total cell protein) bacteria Product can be designed for secretion into the growth media Bacteria Secretes fusion proteins into the Does not express such high levels as E. coli e.g. Staphylococcus growth media aureus Pathogenic Mammalian cells Same biological activity as native proteins Cells can be difficult and expensive to grow Mammalian expression vectors available Cells grow slowly Can be grown in large scale cultures Manipulated cells can be genetically unstable Low productivity as compared to micro-organisms Yeasts Lacks detectable endotoxins Gene expression less easily controlled Generally Regarded As Safe (GRAS) Glycosylation not identical to mammalian systems Fermentation relatively inexpensive Facilitates glycosylation and formation of disulphide bonds Only 0.5% native proteins are secreted so isolation of secreted product is simplified Well established large scale production and downstream processing Cultured insect cells Facilitates glycosylation and Lack of information on glycosylation mechanisms Baculovirus vector formation of disulphide bonds Safe, since few arthropods are adequate Product not always fully functional hosts for baculovirus Baculovirus vector received FDA approval Few differences in functional and antigenic for a clinical trial properties between product and native protein Virus stops host protein amplification. High level expression of product Table 1 (continued)
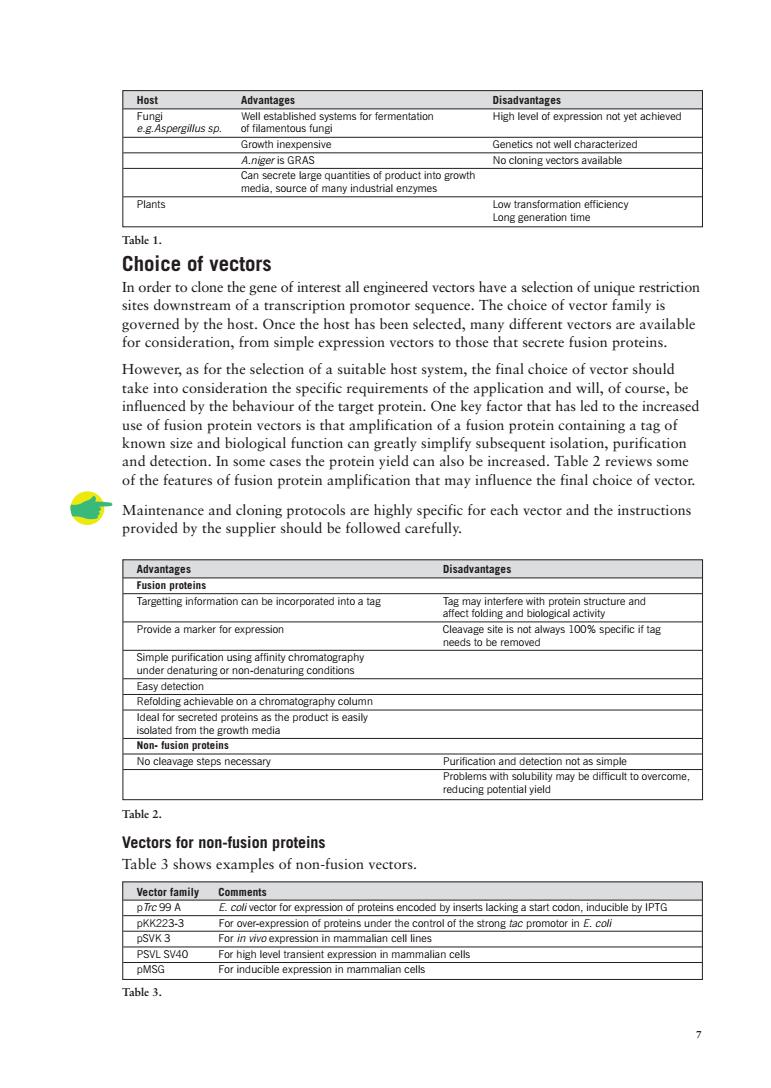
Host Advantages 2g2eegsn&28msoemenaiom High level of expression not vet achieved Growth inexpensive Genetics not well characterized A.nger is G No cloning vectors available Choice of vectors P o sorsideration.rom imple xpio tor tot vailable However,as for the selection of a suitable host system,the final choice of vector should ake ced by the behaviour of the targ rse, sed use of fusic know size dbiological funct aining a and detection In some cases the t otein yield can also ben ased.Table 2r ecific for each vector and the instructions Advantages Disadvantages on p ins Provide a marker for RCngachievabeonachromatograpycolumm g8gepoaasta Non-fusion proteins Table 2. Vectors for non-fusion proteins Table 3 shows examples of non-fusion vectors. ent oded by ins pKK223-3 For over of pro s under the co n in mammalian ce DMSG For ind
7 Advantages Disadvantages Fusion proteins Targetting information can be incorporated into a tag Tag may interfere with protein structure and affect folding and biological activity Provide a marker for expression Cleavage site is not always 100% specific if tag needs to be removed Simple purification using affinity chromatography under denaturing or non-denaturing conditions Easy detection Refolding achievable on a chromatography column Ideal for secreted proteins as the product is easily isolated from the growth media Non- fusion proteins No cleavage steps necessary Purification and detection not as simple Problems with solubility may be difficult to overcome, reducing potential yield Choice of vectors In order to clone the gene of interest all engineered vectors have a selection of unique restriction sites downstream of a transcription promotor sequence. The choice of vector family is governed by the host. Once the host has been selected, many different vectors are available for consideration, from simple expression vectors to those that secrete fusion proteins. However, as for the selection of a suitable host system, the final choice of vector should take into consideration the specific requirements of the application and will, of course, be influenced by the behaviour of the target protein. One key factor that has led to the increased use of fusion protein vectors is that amplification of a fusion protein containing a tag of known size and biological function can greatly simplify subsequent isolation, purification and detection. In some cases the protein yield can also be increased. Table 2 reviews some of the features of fusion protein amplification that may influence the final choice of vector. Maintenance and cloning protocols are highly specific for each vector and the instructions provided by the supplier should be followed carefully. Host Advantages Disadvantages Fungi Well established systems for fermentation High level of expression not yet achieved e.g.Aspergillus sp. of filamentous fungi Growth inexpensive Genetics not well characterized A.niger is GRAS No cloning vectors available Can secrete large quantities of product into growth media, source of many industrial enzymes Plants Low transformation efficiency Long generation time Vectors for non-fusion proteins Table 3 shows examples of non-fusion vectors. Vector family Comments pTrc 99 A E. coli vector for expression of proteins encoded by inserts lacking a start codon, inducible by IPTG pKK223-3 For over-expression of proteins under the control of the strong tac promotor in E. coli pSVK 3 For in vivo expression in mammalian cell lines PSVL SV40 For high level transient expression in mammalian cells pMSG For inducible expression in mammalian cells Table 1. Table 2. Table 3
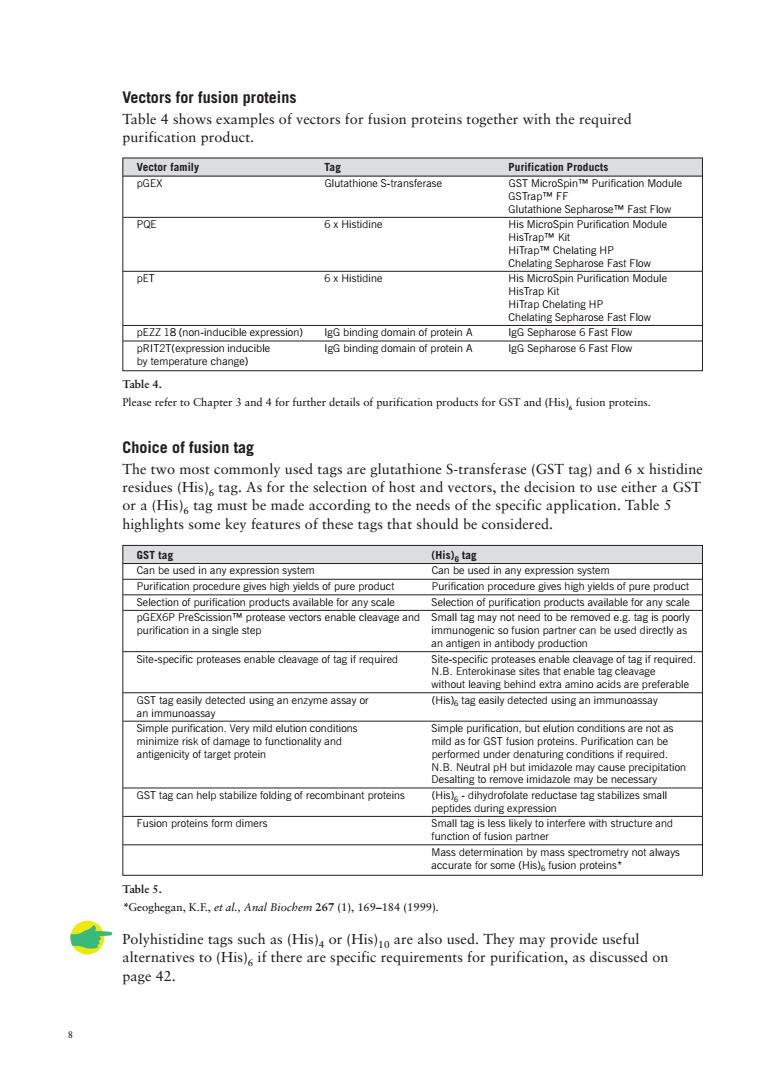
Vectors for fusion proteins Table 4 shows examples of vectors for fusion proteins together with the required purification product. Vector family PGEX athione -transferase POE 6 x Histidine lating HF Fast Flow 6 x Histidine His MicroSpin Purificaion Module e Fast Flow by temperature change) Choice of fusion tag he】 st com ags are gluta athione (Hi 6a %tag mad featurs of thee tags that should be comider GST tag sed in any expression system ure gives hiab vields of pure product Purification procedure aives hiah vields of pure product ourification in a single step Site-specific proteases enable cleavage of tag if required s are preferable but elutio geeeDunctonalyandr STtagcan help protens Fusion proteins form dimers eg。honenterieewhsuctwreand Table 5. "Gcoghegan,K.E.et al,Anal Biocbem 267(1),169-184(1999) Polyhistidine tags such as (His)or (His)are also used.They may provide useful alternatives to (His)if there are specific requirements for purification,as discussed on page 42
8 Choice of fusion tag The two most commonly used tags are glutathione S-transferase (GST tag) and 6 x histidine residues (His)6 tag. As for the selection of host and vectors, the decision to use either a GST or a (His)6 tag must be made according to the needs of the specific application. Table 5 highlights some key features of these tags that should be considered. GST tag (His)6 tag Can be used in any expression system Can be used in any expression system Purification procedure gives high yields of pure product Purification procedure gives high yields of pure product Selection of purification products available for any scale Selection of purification products available for any scale pGEX6P PreScission™ protease vectors enable cleavage and Small tag may not need to be removed e.g. tag is poorly purification in a single step immunogenic so fusion partner can be used directly as an antigen in antibody production Site-specific proteases enable cleavage of tag if required Site-specific proteases enable cleavage of tag if required. N.B. Enterokinase sites that enable tag cleavage without leaving behind extra amino acids are preferable GST tag easily detected using an enzyme assay or (His)6 tag easily detected using an immunoassay an immunoassay Simple purification. Very mild elution conditions Simple purification, but elution conditions are not as minimize risk of damage to functionality and mild as for GST fusion proteins. Purification can be antigenicity of target protein performed under denaturing conditions if required. N.B. Neutral pH but imidazole may cause precipitation Desalting to remove imidazole may be necessary GST tag can help stabilize folding of recombinant proteins (His)6 - dihydrofolate reductase tag stabilizes small peptides during expression Fusion proteins form dimers Small tag is less likely to interfere with structure and function of fusion partner Mass determination by mass spectrometry not always accurate for some (His)6 fusion proteins* Vectors for fusion proteins Table 4 shows examples of vectors for fusion proteins together with the required purification product. Vector family Tag Purification Products pGEX Glutathione S-transferase GST MicroSpin™ Purification Module GSTrap™ FF Glutathione Sepharose™ Fast Flow PQE 6 x Histidine His MicroSpin Purification Module HisTrap™ Kit HiTrap™ Chelating HP Chelating Sepharose Fast Flow pET 6 x Histidine His MicroSpin Purification Module HisTrap Kit HiTrap Chelating HP Chelating Sepharose Fast Flow pEZZ 18 (non-inducible expression) IgG binding domain of protein A IgG Sepharose 6 Fast Flow pRIT2T(expression inducible IgG binding domain of protein A IgG Sepharose 6 Fast Flow by temperature change) Table 4. Please refer to Chapter 3 and 4 for further details of purification products for GST and (His)6 fusion proteins. Table 5. *Geoghegan, K.F., et al., Anal Biochem 267 (1), 169–184 (1999). Polyhistidine tags such as (His)4 or (His)10 are also used. They may provide useful alternatives to (His)6 if there are specific requirements for purification, as discussed on page 42
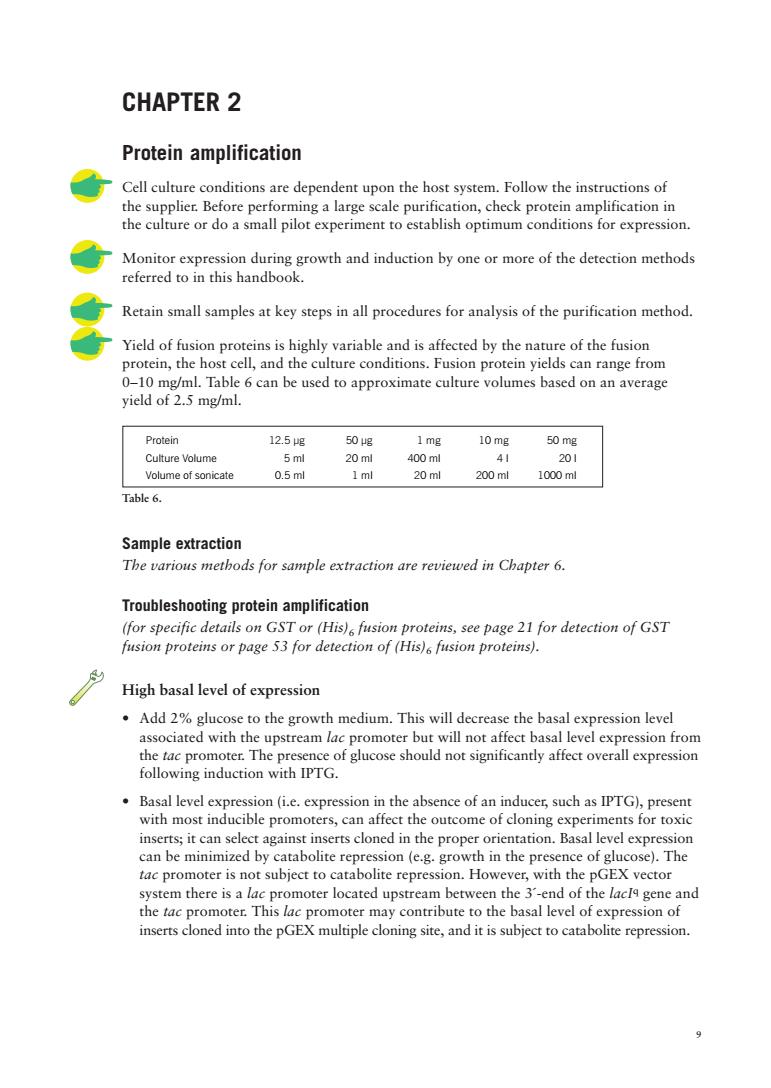
CHAPTER 2 Protein amplification Cell culture conditions are dependent upon the host system.Follow the instructions of the supplier.Bef Monitor Retain small samples at key steps in all procedures for analysis of the purification method. in proteins is highly variable and is affected by the narure of the fion cell,and the culture conditions.Fusion protein yields can range from 0-10 mg/ml.Tabl 6 can be used to approximate culture volumes based on an average yield of 2.5 mg/ml. Protein 12.5g 50g 1mg 10mg 50 mg Culture Volume 400m 41 20 Volume of sonicate 0.5ml 1 ml 20m 200ml 1000ml Table 6. Sample extraction The various metbods for sample extraction are reviewed in Chapter6 Troubleshooting protein amplification (for specific details on GST or (His)fusion proteins,see page 21 for detection of GST fusion proteins or page 53 for detection of (His)fusion proteins). High basal level of expression Add 2%glucose to the growth medium.This will decrease the basal expression level associated with the upstream lac promoter but will not affect basal level expression from the tac promoter.The presence of glucose should not significantly affect overall expression following induction with IPTG. Basal level expression (i.e.expression in the absence of an inducer,such as IPTG),present with most inducible promoters,can affect the outcome of cloning experiments for toxic inserts;it can select against inserts cloned in the proper orientation.Basal level expression can be minimized by catabolite repression (e.g.growth in the presence of glucose).The tac promoter is not subject to catabolite repression.However,with the pGEX vector system there is a lac promoter located upstream between the 3'-end of the lacla gene and the tac promoter.This lac promoter may contribute to the basal level of expression of inserts cloned into the pGEX multiple cloning site,and it is subject to catabolite repression
9 CHAPTER 2 Protein amplification Cell culture conditions are dependent upon the host system. Follow the instructions of the supplier. Before performing a large scale purification, check protein amplification in the culture or do a small pilot experiment to establish optimum conditions for expression. Monitor expression during growth and induction by one or more of the detection methods referred to in this handbook. Retain small samples at key steps in all procedures for analysis of the purification method. Yield of fusion proteins is highly variable and is affected by the nature of the fusion protein, the host cell, and the culture conditions. Fusion protein yields can range from 0–10 mg/ml. Table 6 can be used to approximate culture volumes based on an average yield of 2.5 mg/ml. Protein 12.5 µg 50 µg 1 mg 10 mg 50 mg Culture Volume 5 ml 20 ml 400 ml 4 l 20 l Volume of sonicate 0.5 ml 1 ml 20 ml 200 ml 1000 ml Sample extraction The various methods for sample extraction are reviewed in Chapter 6. Troubleshooting protein amplification (for specific details on GST or (His)6 fusion proteins, see page 21 for detection of GST fusion proteins or page 53 for detection of (His)6 fusion proteins). High basal level of expression • Add 2% glucose to the growth medium. This will decrease the basal expression level associated with the upstream lac promoter but will not affect basal level expression from the tac promoter. The presence of glucose should not significantly affect overall expression following induction with IPTG. • Basal level expression (i.e. expression in the absence of an inducer, such as IPTG), present with most inducible promoters, can affect the outcome of cloning experiments for toxic inserts; it can select against inserts cloned in the proper orientation. Basal level expression can be minimized by catabolite repression (e.g. growth in the presence of glucose). The tac promoter is not subject to catabolite repression. However, with the pGEX vector system there is a lac promoter located upstream between the 3´-end of the lacIq gene and the tac promoter. This lac promoter may contribute to the basal level of expression of inserts cloned into the pGEX multiple cloning site, and it is subject to catabolite repression. Table 6