
现代有机合成化学 petr用oo 复杂分子合成设计与全合成( Tamiflu:An Anti-influenza Drug 姚祝军 南京大学化学化工学院 194 Email:yaoz@nju.edu.cn 2015年5月 Tamiflu (Oseltamivir Phosphate) Neuraminidase inhibitors design ered at Glead 1995 eated se of 53 Tamiflu(达菲) Synthesis of Tamiflu(to 2010) a Roche amo L Chom Re:200910439
1 姚祝军 南京大学化学化工学院 Email: yaoz@nju.edu.cn 2015年5月 1 现代有机合成化学 复杂分子合成设计与全合成 (7) 南京大学化学化工学院 2014级研究生课程 Tamiflu: An Anti-influenza Drug Oseltamivir, an antiviral drug, slows the spread of influenza (flu) virus between cells in the body by stopping the virus from chemically cutting ties with its host cell; median time to symptom alleviation is reduced by 0.5–1 day. The drug is marketed under the trade name Tamiflu, and is taken orally in capsules or as a suspension. It has been used to treat and prevent influenza A virus and influenza B virus infection in over 50 million people since 1999. Oseltamivir is a prodrug, a (relatively) inactive chemical which is converted into its active form by metabolic process after it is taken into the body. It was the first orally active neuraminidase inhibitor commercially developed. It was developed by C.U. Kim, W. Lew, and X. Chen of US-based Gilead Sciences, and is marketed by Genentech. In Japan, it is marketed by Chugai Pharmaceutical Co. As of December 15, 2010, WHO reported 314 samples of the prevalent 2009 pandemic H1N1 flu tested worldwide have shown resistance to oseltamivir. http://en.wikipedia.org/wiki/Oseltamivir © 2016 YZJ@NJU Tamiflu (Oseltamivir Phosphate) Influenza Seasonal Avian Swine Discovered at Gilead 1995 Codeveloped with Roche Launched by Roche 1999 50 million people treated $2.1 billion in 2006 $564 million in 2008 Predicted sales increase of 531% 3 © 2016 YZJ@NJU Neuraminidase inhibitors design 4 © 2016 YZJ@NJU Tamiflu (达菲) Orally active neuraminidase inhibitor Competitive inhibitor towards sialic acid Designed as analogue of oxocarbonium intermed. Prevents viral release and spread of disease Dose: 75 mg twice daily for 5 days 2010- Production at 400 million packs per year 5 Magano, J. Chem. Rev. 2009, 109, 4398-4438. © 2016 YZJ@NJU Synthesis of Tamiflu (to 2010) Over 30 published syntheses of molecule or intermediates (up to 2010) First synthesis by Gilead in 1997 A process route by Hoffmann-La Roche Inexpensive route by Shibasaki Shortest synthesis by Trost; and highest yielding synthesis by Hayashi and Dawei Ma 6 Recent reviews, see: Tamiflu: The Supply Problem Farina, V.; Brown, J. D. Angew. Chem. Int. Ed. 2006, 45, 7330–7334 Synthetic Strategies for Oseltamivir Phosphate Shibasaki, M.; Kanai, M. Eur. J. Org. Chem. 2008, 1839-1850. Magano, J. Chem. Rev. 2009, 109, 4398-4438
Synthesis Strategies Shikimic acid as the starting material.The chiron approach m 积a 7得方 sSythesisilead Roche's proce forprodcn 00H 密器能 m Shi's two optimizations First Synthesis-Gilead Chiron approach using as starting materia 滋器器之 Uses azide ch cale as candidate sL无送:t0og6rn2009,743970:r200920i4. 2
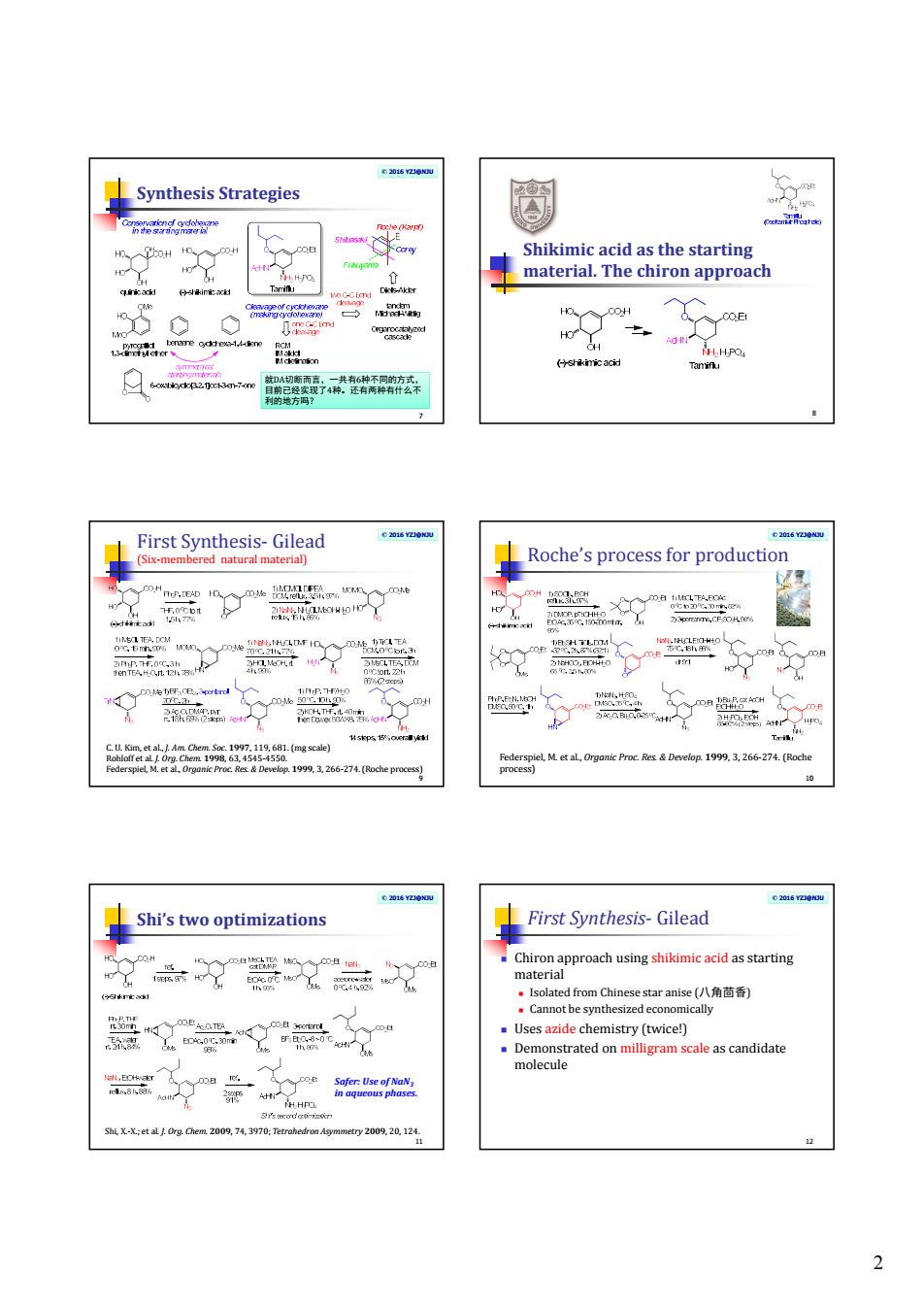
2 © 2016 YZJ@NJU Synthesis Strategies 7 就DA切断而言,一共有6种不同的方式, 目前已经实现了4种。还有两种有什么不 利的地方吗? Shikimic acid as the starting material. The chiron approach 8 © 2016 YZJ@NJU First Synthesis- Gilead (Six-membered natural material) 9 C. U. Kim, et al., J. Am. Chem. Soc. 1997, 119, 681. (mg scale) Rohloff et al. J. Org. Chem. 1998, 63, 4545-4550. Federspiel, M. et al., Organic Proc. Res. & Develop. 1999, 3, 266-274. (Roche process) © 2016 YZJ@NJU Roche’s process for production 10 Federspiel, M. et al., Organic Proc. Res. & Develop. 1999, 3, 266-274. (Roche process) © 2016 YZJ@NJU Shi’s two optimizations 11 Shi, X.-X.; et al. J. Org. Chem. 2009, 74, 3970; Tetrahedron Asymmetry 2009, 20, 124. Safer: Use of NaN3 in aqueous phases. © 2016 YZJ@NJU First Synthesis- Gilead Chiron approach using shikimic acid as starting material Isolated from Chinese star anise (八角茴香) Cannot be synthesized economically Uses azide chemistry (twice!) Demonstrated on milligram scale as candidate molecule 12
Starting materiaLshikimic acid Azide:an explosive reagent the y car airbag systems.It is Dcoitepbierdodiiteisofoayimited e Process Synthesis-Hofmann-La Roche Process Synthesis-Hoffmann-La Roche up and the te◇ 学学习 062051epL4 Process Synthesis-Hoffmann-La Roche Roche azide-free synthesis 。35%from epoxide Uses shikimic acid as starting material 。Azide-ree proces .Avoids chromatography 3
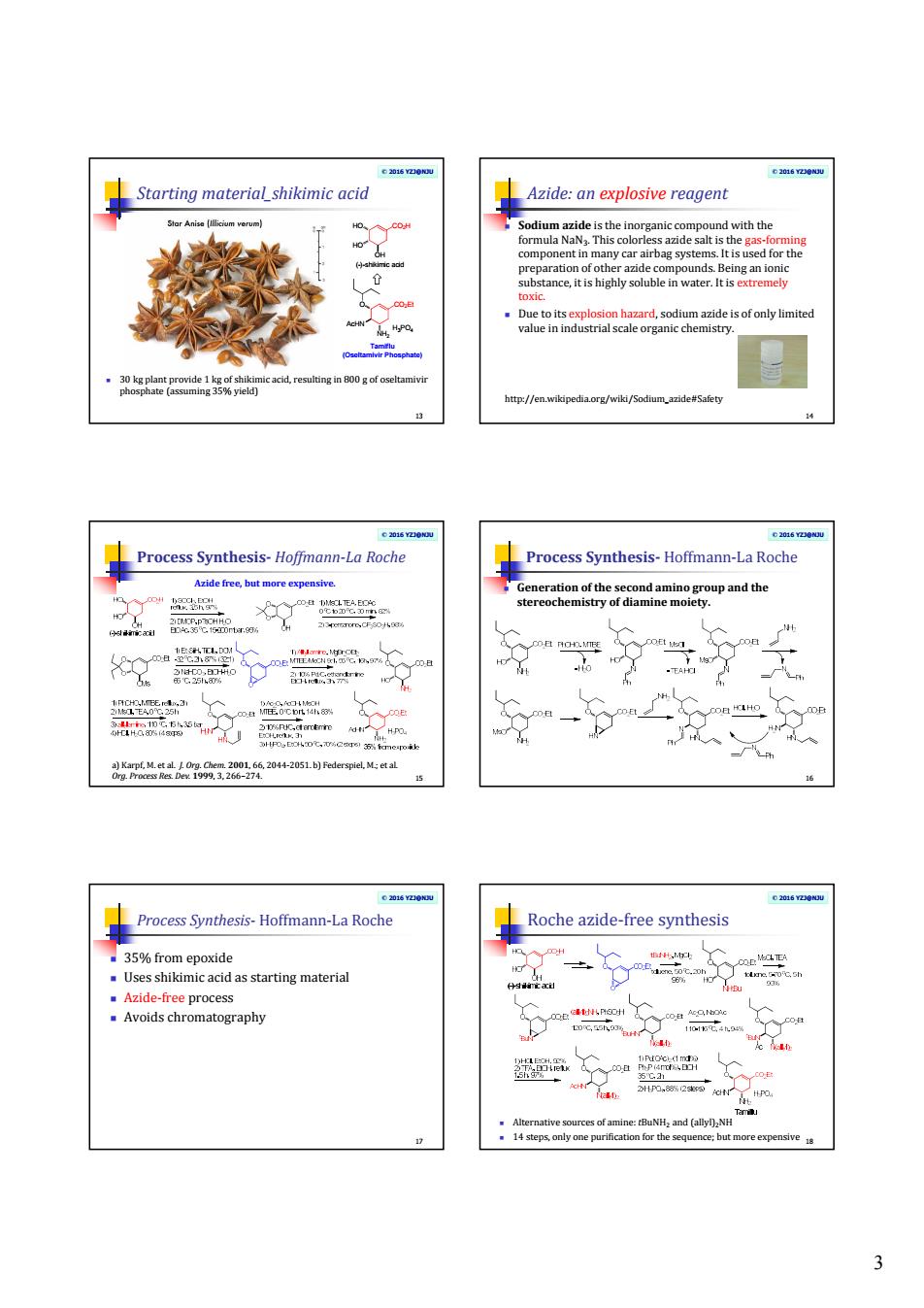
3 © 2016 YZJ@NJU Starting material_shikimic acid 30 kg plant provide 1 kg of shikimic acid, resulting in 800 g of oseltamivir phosphate (assuming 35% yield) 13 O NH2 AcHN CO2Et H3PO4 Tamiflu (Oseltamivir Phosphate) HO HO OH CO2H (-)-shikimic acid © 2016 YZJ@NJU Azide: an explosive reagent Sodium azide is the inorganic compound with the formula NaN3 . This colorless azide salt is the gas-forming component in many car airbag systems. It is used for the preparation of other azide compounds. Being an ionic substance, it is highly soluble in water. It is extremely toxic. Due to its explosion hazard, sodium azide is of only limited value in industrial scale organic chemistry. 14 http://en.wikipedia.org/wiki/Sodium_azide#Safety © 2016 YZJ@NJU Process Synthesis- Hoffmann-La Roche 15 a) Karpf, M. et al. J. Org. Chem. 2001, 66, 2044-2051. b) Federspiel, M.; et al. Org. Process Res. Dev. 1999, 3, 266–274. Azide free, but more expensive. © 2016 YZJ@NJU Process Synthesis- Hoffmann-La Roche Generation of the second amino group and the stereochemistry of diamine moiety. 16 © 2016 YZJ@NJU Process Synthesis- Hoffmann-La Roche 35% from epoxide Uses shikimic acid as starting material Azide-free process Avoids chromatography 17 © 2016 YZJ@NJU Roche azide-free synthesis Alternative sources of amine: tBuNH2 and (allyl)2NH 14 steps, only one purification for the sequence; but more expensive 18
Other cyclohexanes as the starting material(desymmetrization) = Roche desymmetrization strategy 集Shbasak's yntheis 5 steps, 0% t al 1.6-dimethox .key steps:cis re irality: 三2高“文禁文 力受力二 hibasal.asoe o Shibasakl's second synthesis Shibasaki's synthesis 只三2 三需之 20 steps7%(2 synthesis) starting materia 警文要二. .Shibaak.M.etal Orp.Let 007.9.259-762 4
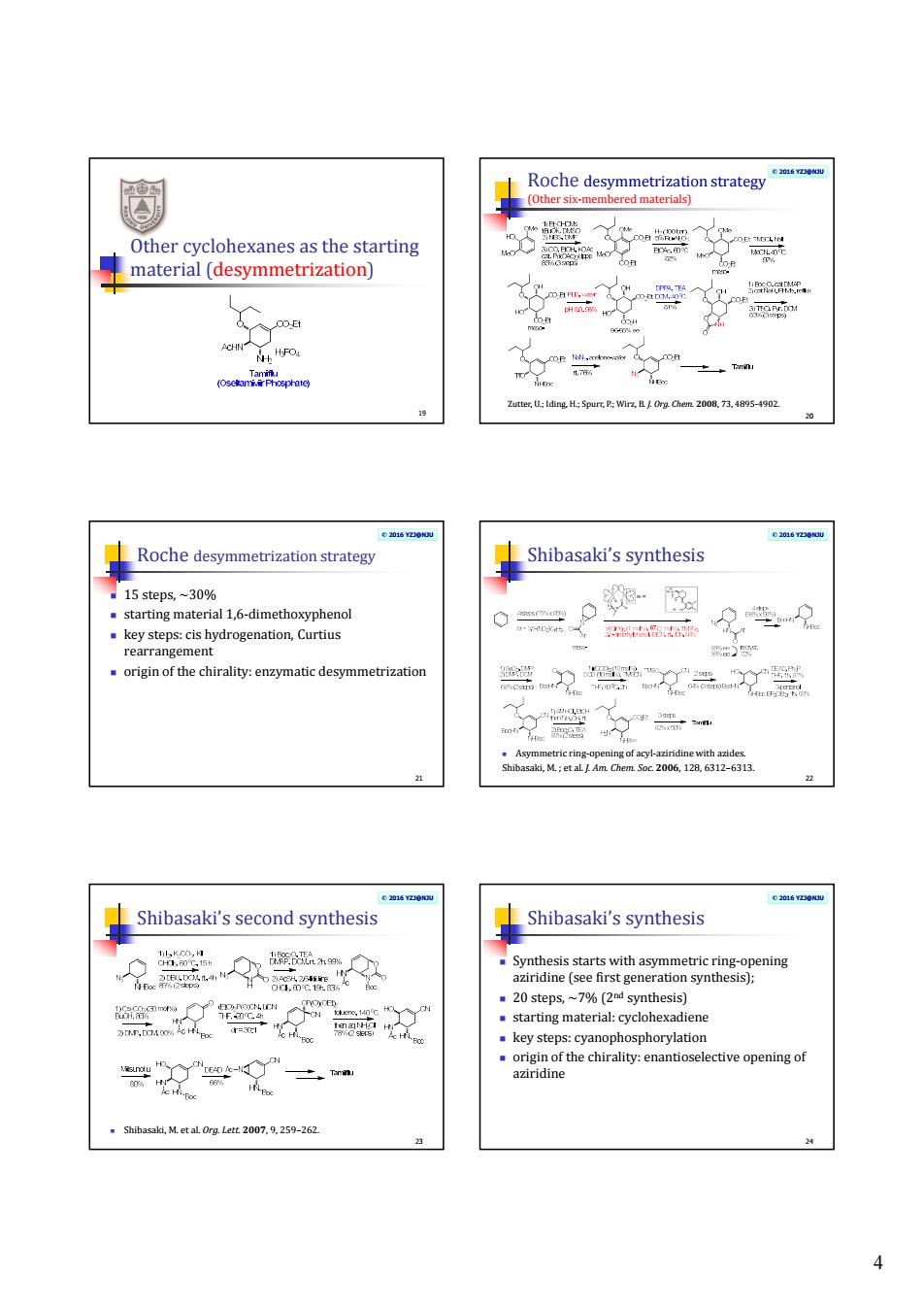
4 Other cyclohexanes as the starting material (desymmetrization) 19 © 2016 YZJ@NJU Roche desymmetrization strategy (Other six-membered materials) 20 Zutter, U.; Iding, H.; Spurr, P.; Wirz, B. J. Org. Chem. 2008, 73, 4895-4902. © 2016 YZJ@NJU Roche desymmetrization strategy 15 steps, ~30% starting material 1,6-dimethoxyphenol key steps: cis hydrogenation, Curtius rearrangement origin of the chirality: enzymatic desymmetrization 21 © 2016 YZJ@NJU Shibasaki’s synthesis Asymmetric ring-opening of acyl-aziridine with azides. Shibasaki, M. ; et al. J. Am. Chem. Soc. 2006, 128, 6312–6313. 22 © 2016 YZJ@NJU Shibasaki’s second synthesis Shibasaki, M. et al. Org. Lett. 2007, 9, 259–262. 23 © 2016 YZJ@NJU Shibasaki’s synthesis Synthesis starts with asymmetric ring-opening aziridine (see first generation synthesis); 20 steps, ~7% (2nd synthesis) starting material: cyclohexadiene key steps: cyanophosphorylation origin of the chirality: enantioselective opening of aziridine 24
Trost's short route ot Novp-c ·Scale7cost7 M Trost,TZhang Ango Chem n079. 陶 Metathesis approach by Yao and Cong One C-C bond cleavage: Construction of the cyclohexane 2 oum) Cong Yao Ong Chem 2006.735165. Yao-RCM 。8%yield in25 steps starting 验学文受 法6器sa。 5
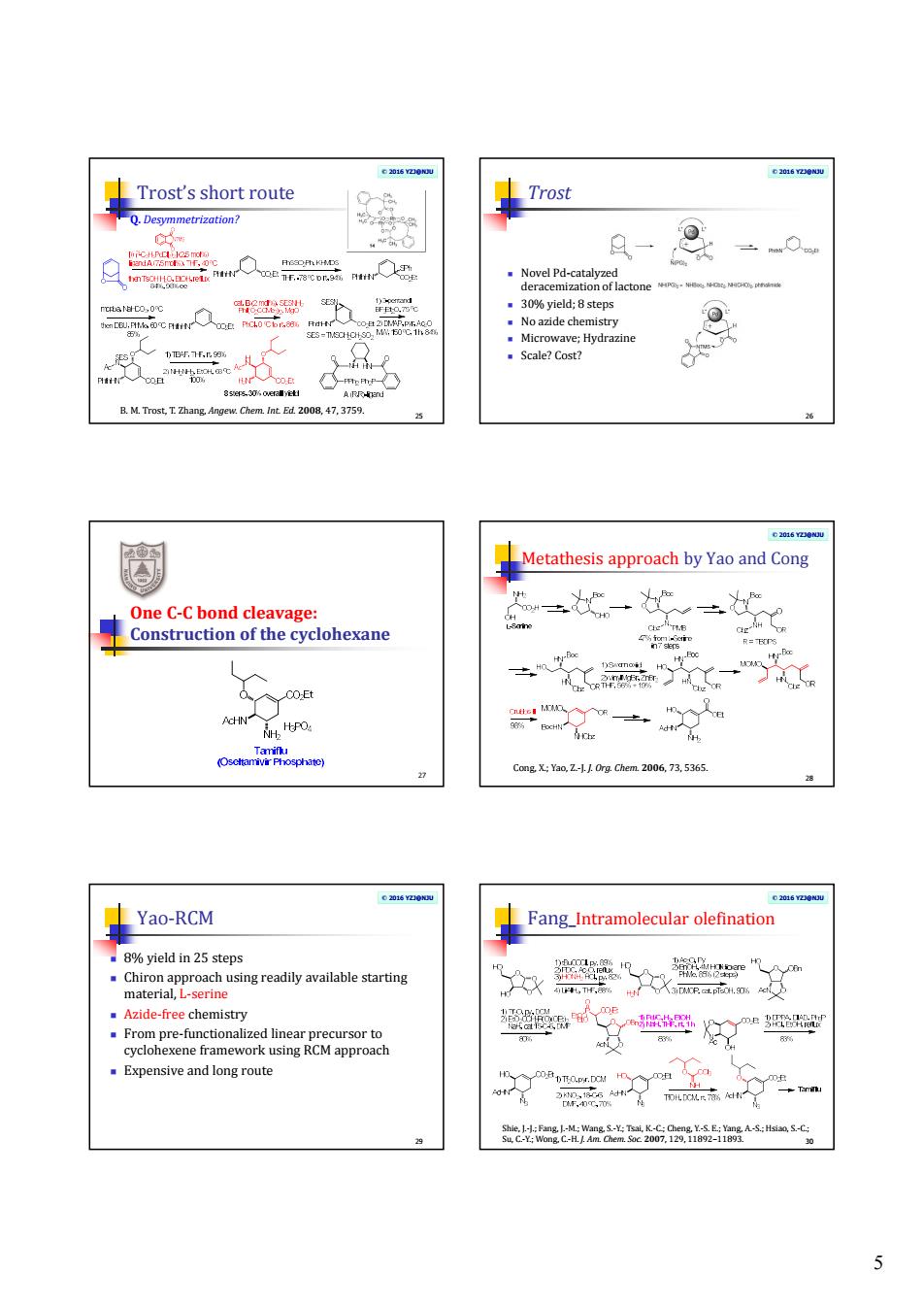
5 © 2016 YZJ@NJU Trost’s short route 25 B. M. Trost, T. Zhang, Angew. Chem. Int. Ed. 2008, 47, 3759. Q. Desymmetrization? © 2016 YZJ@NJU Trost Novel Pd-catalyzed deracemization of lactone 30% yield; 8 steps No azide chemistry Microwave; Hydrazine Scale? Cost? 26 One C-C bond cleavage: Construction of the cyclohexane 27 © 2016 YZJ@NJU Metathesis approach by Yao and Cong 28 Cong, X.; Yao, Z.-J. J. Org. Chem. 2006, 73, 5365. © 2016 YZJ@NJU Yao-RCM 8% yield in 25 steps Chiron approach using readily available starting material, L-serine Azide-free chemistry From pre-functionalized linear precursor to cyclohexene framework using RCM approach Expensive and long route 29 © 2016 YZJ@NJU Fang_Intramolecular olefination 30 Shie, J.-J.; Fang, J.-M.; Wang, S.-Y.; Tsai, K.-C.; Cheng, Y.-S. E.; Yang, A.-S.; Hsiao, S.-C.; Su, C.-Y.; Wong, C.-H. J. Am. Chem. Soc. 2007, 129, 11892–11893
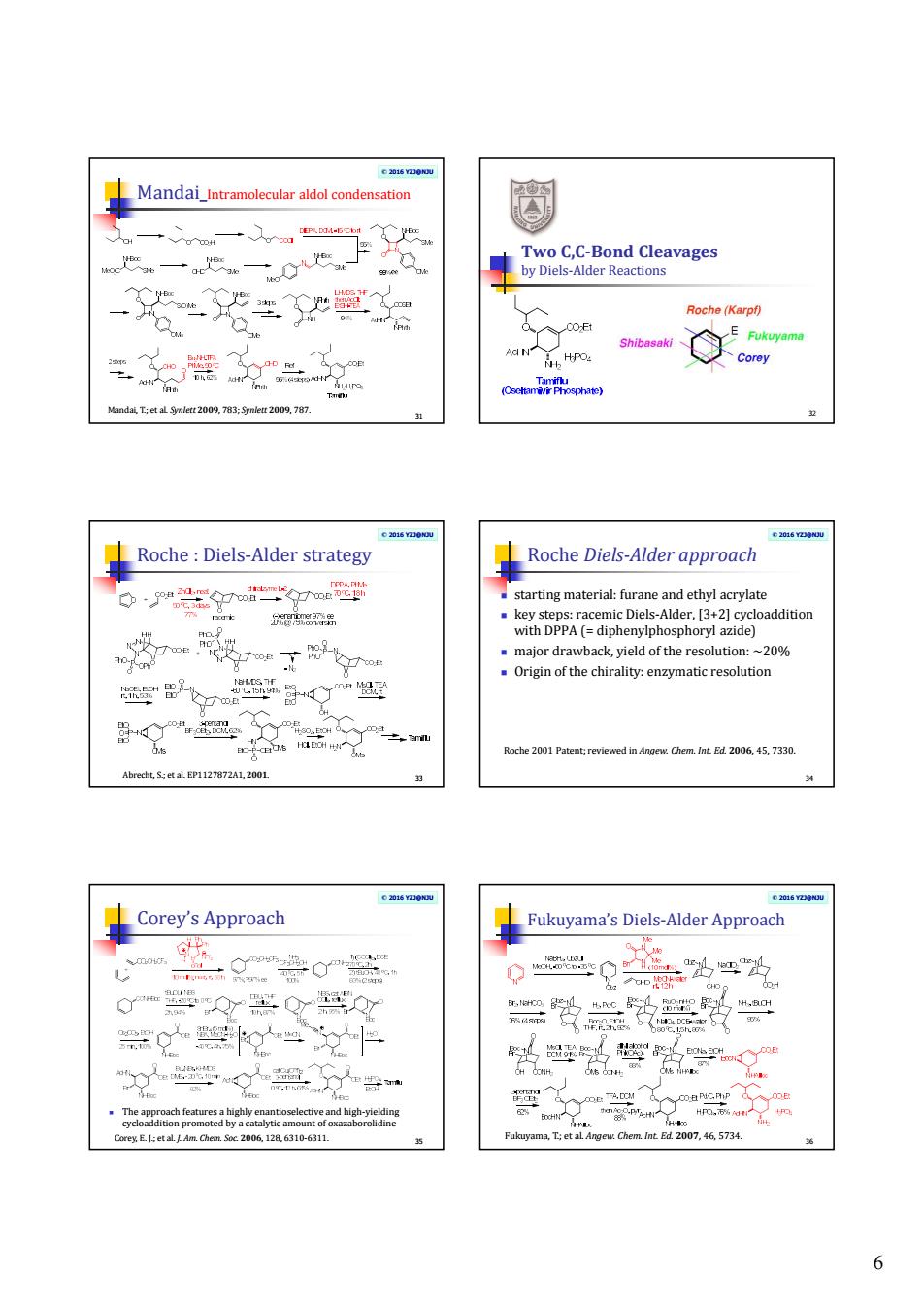
Roche(Karpl) 生授之兰之 hn玉林ge20的:o20的m Roche:Diels-Alder strategy Roche Diels-Aldeapproach 0p型 。ade2 2 为 器堂子燮 g品这之m am.t206,45.7330 Abnc,E127872A1,201 Corey's Approach Fukuyama'Diels-Alder Approach 文尝心景 0品父=出为 悬 头克警的 6
6 © 2016 YZJ@NJU Mandai_Intramolecular aldol condensation 31 Mandai, T.; et al. Synlett 2009, 783; Synlett 2009, 787. Two C,C-Bond Cleavages by Diels-Alder Reactions 32 © 2016 YZJ@NJU Roche : Diels-Alder strategy 33 Abrecht, S.; et al. EP1127872A1, 2001. © 2016 YZJ@NJU Roche Diels-Alder approach starting material: furane and ethyl acrylate key steps: racemic Diels-Alder, [3+2] cycloaddition with DPPA (= diphenylphosphoryl azide) major drawback, yield of the resolution: ~20% Origin of the chirality: enzymatic resolution 34 Roche 2001 Patent; reviewed in Angew. Chem. Int. Ed. 2006, 45, 7330. © 2016 YZJ@NJU Corey’s Approach The approach features a highly enantioselective and high-yielding cycloaddition promoted by a catalytic amount of oxazaborolidine 35 Corey, E. J.; et al. J. Am. Chem. Soc. 2006, 128, 6310-6311. © 2016 YZJ@NJU Fukuyama’s Diels-Alder Approach 36 Fukuyama, T.; et al. Angew. Chem. Int. Ed. 2007, 46, 5734
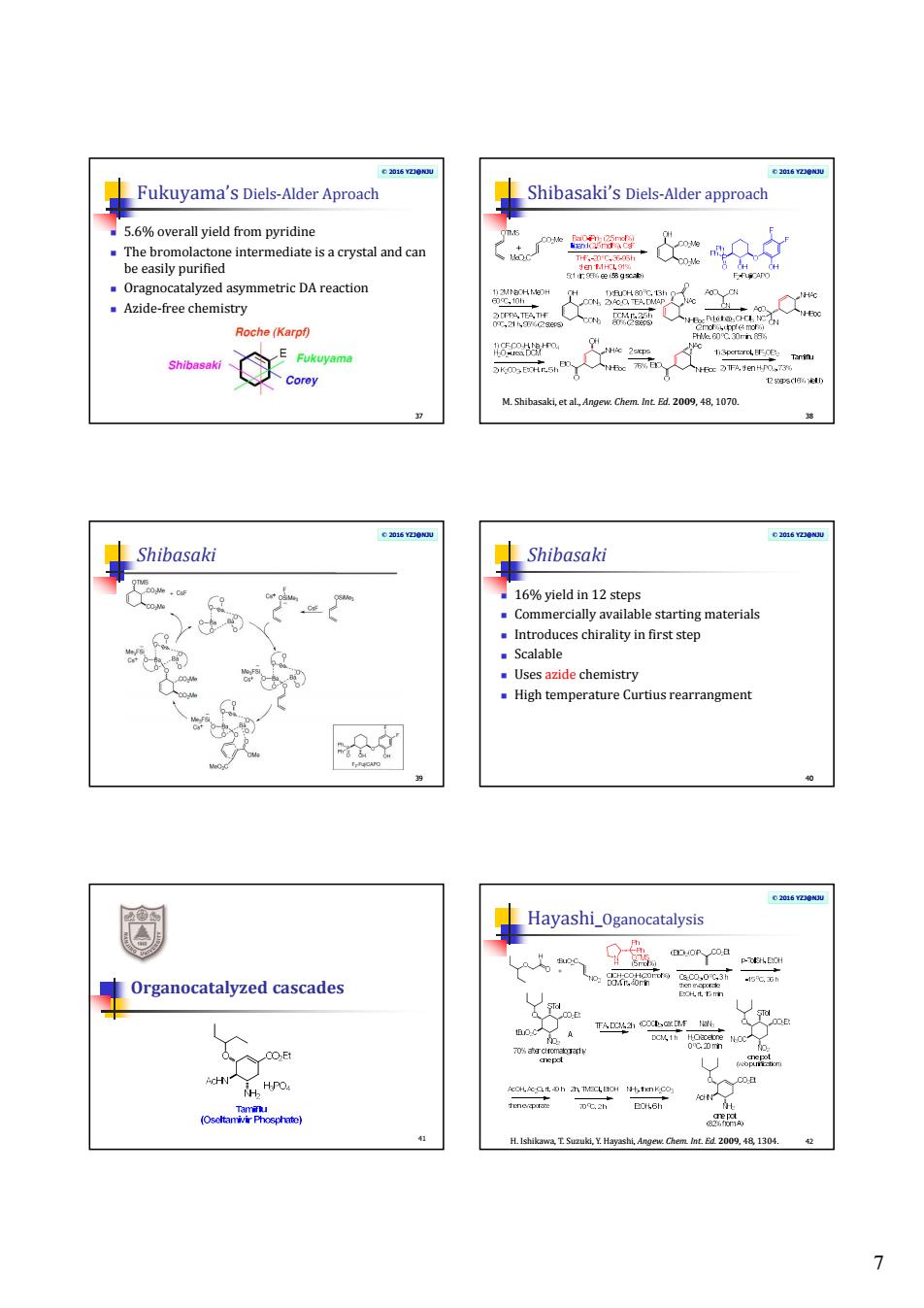
Fukuyama's Diels-Alder Aproach Shibasak's Delderpc is a crystal and can .Oragnocatalyzed asymmetric DAreaction Azide-free chemistry Roche(Karp时 M.Shibsakiet alAngee Chem 1070. ⊥Shibasaki Shibasaki .o 16%vield in 12 s .Introduces chiralityin first step Scalable mistry rtius rearrangment Hayashi_Oganocatalysis Organocatalyzed cascades HPO 7
7 © 2016 YZJ@NJU Fukuyama’s Diels-Alder Aproach 5.6% overall yield from pyridine The bromolactone intermediate is a crystal and can be easily purified Oragnocatalyzed asymmetric DA reaction Azide-free chemistry 37 © 2016 YZJ@NJU Shibasaki’s Diels-Alder approach 38 M. Shibasaki, et al., Angew. Chem. Int. Ed. 2009, 48, 1070. © 2016 YZJ@NJU Shibasaki 39 © 2016 YZJ@NJU Shibasaki 16% yield in 12 steps Commercially available starting materials Introduces chirality in first step Scalable Uses azide chemistry High temperature Curtius rearrangment 40 Organocatalyzed cascades 41 © 2016 YZJ@NJU Hayashi_Oganocatalysis H. Ishikawa, T. Suzuki, Y. Hayashi, Angew. Chem. Int. Ed. 2009, 48, 1304. 42
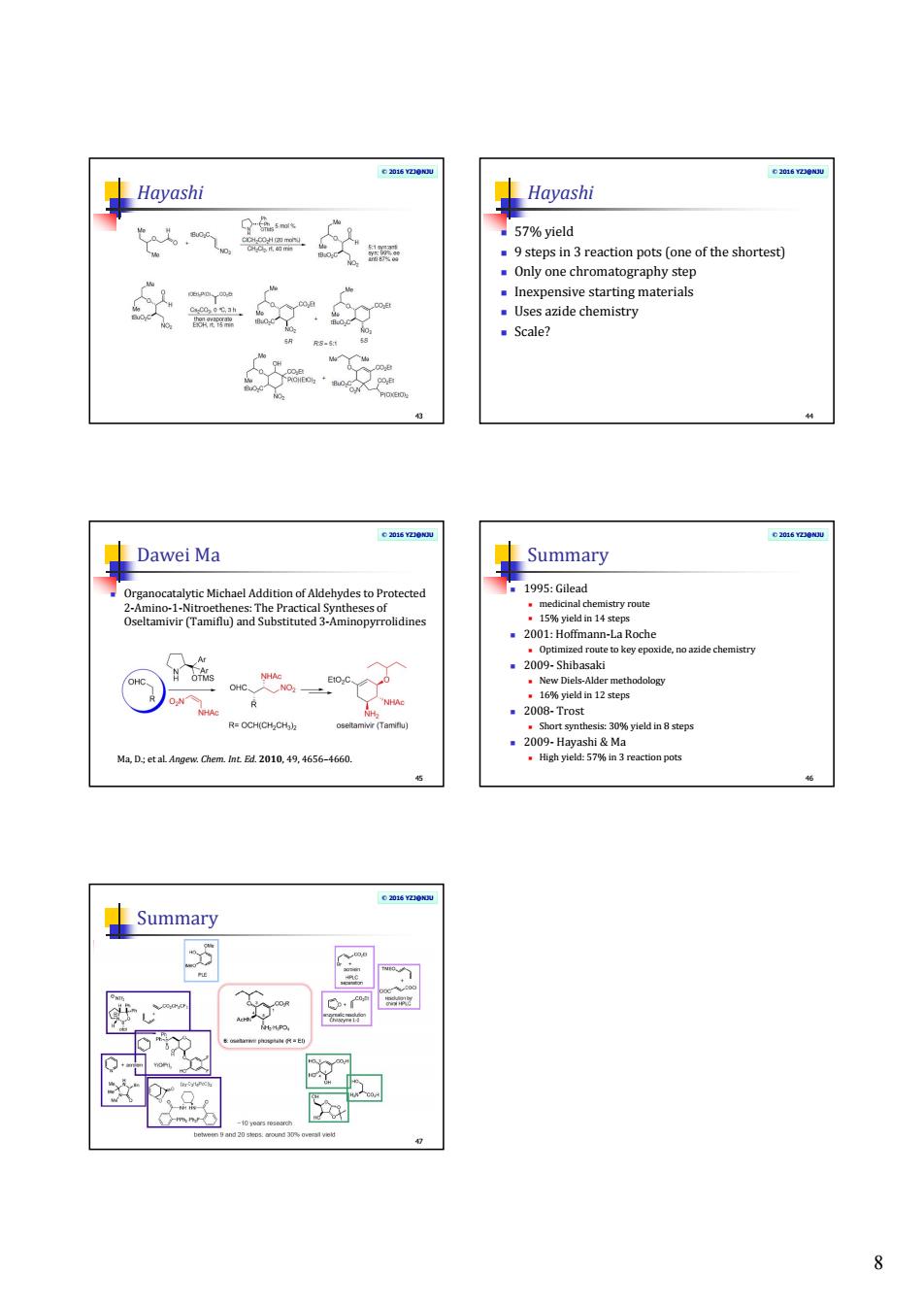
器头 57%yleld pots (on of the shortest) 头燕 materials Uses azide chemistry Scale? 5 Dawei Ma ydestoProtected 1995:Gilead e Summary 8
8 © 2016 YZJ@NJU Hayashi 43 © 2016 YZJ@NJU Hayashi 57% yield 9 steps in 3 reaction pots (one of the shortest) Only one chromatography step Inexpensive starting materials Uses azide chemistry Scale? 44 © 2016 YZJ@NJU Dawei Ma Organocatalytic Michael Addition of Aldehydes to Protected 2-Amino-1-Nitroethenes: The Practical Syntheses of Oseltamivir (Tamiflu) and Substituted 3-Aminopyrrolidines45 Ma, D.; et al. Angew. Chem. Int. Ed. 2010, 49, 4656–4660. © 2016 YZJ@NJU Summary 1995: Gilead medicinal chemistry route 15% yield in 14 steps 2001: Hoffmann-La Roche Optimized route to key epoxide, no azide chemistry 2009- Shibasaki New Diels-Alder methodology 16% yield in 12 steps 2008- Trost Short synthesis: 30% yield in 8 steps 2009- Hayashi & Ma High yield: 57% in 3 reaction pots 46 © 2016 YZJ@NJU Summary 47