
型 教师联系方式 姚祝军 现代有机合成I(B1) ·办公室:仙林化学楼E515 姚祝军 南京大学化学化工学院 ,课件下载 Email:yaoz@nju.edu.cn http://hysz.nju.edu.cn/yaozj/resources.htm 2016年4月 十课程内容提要 复杂分子合成发展历史 基本概含 目标分子考 机合动 5 创造/生产 Endless endeavor 化学质的合与格 海葵毒素:化学合成的最复杂分子 春天然一创选物质(原 21210 :冷@ 先进的方法与策略
1 现代有机合成 II (B1) 姚祝军 南京大学化学化工学院 Email: yaoz@nju.edu.cn 2016年4月 1 南京大学化学化工学院 2015级研究生课程 © 2016 YZJ@NJU 教师联系方式 姚祝军 办公室:仙林化学楼E515 电话:89683727 (office); 89683732 (lab) 电子邮件:yaoz@nju.edu.cn 课件下载 http://hysz.nju.edu.cn/yaozj/resources.htm 2 © 2016 YZJ@NJU 课程内容提要 1. 复杂分子合成发展历史 2. 基本概念 3. 目标分子考察 4. 反合成分析 5. 合成原料选择 6. 路线考察 合成经济性、选择性与合成效率 7. 复杂分子合成范例分析 8. 类天然产物化学 3 © 2016 YZJ@NJU 4 有机合成是在一个真实世界的旁边创造着一个新的世界。 - R. B. Woodward,1965 “. for his outstanding achievements in the art of organic synthesis ." Research Scope 天然产物 非天然分子 材料用途分子 生物活性分子 理论研究分子 医学用途分子 © 2016 YZJ@NJU 创造/生产 5 1. 化学物质的合成与制备 • 天然-人工复制 • 非天然-创造物质(源) 2. 创造具有各种功能的物质 • 社会发展进步的物质基础 • 设计合成与应用紧密联系 • 结构与性质的关联性 3. 先进的方法与策略 • Economy, efficiency, elegance • Previously undreamed complexity © 2016 YZJ@NJU Endless endeavor 6 Palytoxin C131H227N3O53 MW 2692.26 64 asymmetric 6 Olefin centers 2 70=1.2x1021 isomers Tailored Synthesis: Chirality Rational logic driven design High level of sophistication Nozaki-Kishi Coupling 海葵毒素: 化学合成的最复杂分子

上有机合成产品 有机合成=分子工程 药物工业产品 分子 用化 杀虫刑 除草剂 路贵 060002, e Increasing numbers of publications 奎宁(Quinine) rganic natural product synthesi 童宁的发现与药用价值 表童宁的分离、种植和商业化 16年,基督教传教士(神父)Calancha描迷了知何使用室 含轻”宝微 没有 ”象马景头雀务 ”年,有兰在a果国生产2万骑奎字,古世界市场的 2
2 © 2016 YZJ@NJU 有机合成产品 药物工业产品. 家用化学品. 农用化学品: 杀虫剂、除草剂. 高分子聚合物及其单体. 粘结剂、表面涂料. 染料. 香精香料. 等等. 7 Dreamliner (梦想客机) 波音787大量采用复合材料。使用物料(按重量)61% 复合物料 (碳纤维),20% 铝,11% 钛,8% 钢。按体积,占787全机物料 的80%均为复合物料。 低燃料消耗、较低的污染排放、高效益及舒适的客舱环境,可实 现更多的点对点不经停直飞航线。以及较低噪音、较高可靠度、 较低维修成本。 波音787系列可覆盖6500至16000公里。 © 2016 YZJ@NJU 有机合成 = 分子工程 有机合成: 利用创造性的化学转化实现具有特定化学结构的分 子与分子集结体的人工获取。 8 分子 合成 策略 合成 方法学 复杂性 + 多样性 精准性 © 2016 YZJ@NJU Increasing numbers of publications Number of papers in the general area of organic & natural product synthesis from 1905-1979: ~50,000 (74 years) from 1980-1992: ~47,000 (12 years) from 1993-2005: ~90,000 (12 years) 9 © 2016 YZJ@NJU 奎宁(Quinine) 首次分离获得: 1820 分子量推定: 1854 结构测定: 1908 第一次实验室人工合成: 1944 第一次对映体选择性合成: 2001 (Why did it?) 10 http://en.wikipedia.org/wiki/Quinine © 2016 YZJ@NJU 奎宁的发现与药用价值 1633年,基督教传教士(神父) Calancha 描述了如何使用奎 宁树皮治发烧; 1645年,教父Bartolome Tafur带了一些奎宁树皮去罗马给 许多神职人员使用;枢机主教John de Lugo写了使用说明的 小册子和树皮一起散发,使奎宁树皮的使用变得普遍。 1655年罗马教皇的秘密会议上,确认没有人再死于疟疾; 1654 年,英国人开始知道如何使用奎宁树皮; 1735年,法国植物学家Joseph de Jussieu远航到南美洲,发 现和描述了奎宁树皮的源植物,他送样品到瑞典。1739年, Carl Linneaus 命名属金鸡纳树。金鸡纳属有20至40种 ,种 间很难分辨,大多是下层树木。 11 © 2016 YZJ@NJU 奎宁的分离、种植和商业化 1820年,法国化学家 Joseph Pelletier 和Joseph Caventou 从 树皮中分离获得了生物碱-奎宁,并确定它是在秘鲁树皮 的活性成分; 1861年,澳大利亚人Charles Ledger从一个名叫Manuel Incra的艾马拉印第安人那里获得了植物种子; 1930年,荷兰在Java果园生产22万磅奎宁,占世界市场的 97%。 12

奎宁的化学性质 疟疾治疗药物 套种粉未,无气味,但口味极: “套宝是一种药用天然产物,用于治疗心律失常以及 : 厄瓜多尔,1944年 集套宁化学合成 tH c98 6o Resolution of Chirality Degradation LouisPasteur(1853) aul Rabe (1909) 2是-是 P.Rabe.Anmalen.1909.365.366. 3
3 © 2016 YZJ@NJU 奎宁的化学性质 奎宁是一种白色粉末,无气味,但口味极苦; 作为调味剂使用. 奎宁是一种药用天然产物, 用于治疗心律失常以及 疟疾; 奎宁通过抑制疟原虫繁殖防止疟疾; 治疗一些与疟疾有关的发烧和疼痛; 奎宁是一种荧光材料 在紫外灯下会发出荧光。 © 2016 YZJ@NJU 疟疾治疗药物 影响世界历史进程的分子:在第二次世界大战期间,德国 人征服了荷兰,日本控制了菲律宾和印度尼西亚,同盟国 的奎宁供应被切断。数以万计在非洲和南太平洋地区作战 的美军由于缺乏奎宁而死亡。1944年,哈佛大学化学系 R. B. Woodward教授在实验室成功合成奎宁。 14 © 2016 YZJ@NJU 厄瓜多尔,1944年 15 太阳下晒着的金鸡纳树皮 © 2016 YZJ@NJU 奎宁化学合成 William Henry Perkin & August Wilhelm Hoffman (1856) 16 S. Garfield, Mauve, Faber and Faber, London, 2000, p224. O. Meth-Cohn, M. Smith, J. Chem. 苯胺紫 Soc. Perkin Trans. 1, 2004, 5. © 2016 YZJ@NJU Resolution of Chirality 17 Louis Pasteur (1853) L. Pasteur, Annalen, 1853, 88, 209. © 2016 YZJ@NJU Degradation 18 P. Rabe, Annalen, 1909, 365, 366. Paul Rabe (1909)
Reconstitution Rabe,1918 集Skr3p隆琳合成法 R.H.F.Manske,M.Kulka,Org.React.1953,7.59-98. The first(formal)total synthesis 泰雪来-醉 3-5场 anma& SET oxidation Enantioselective total synthesis 立263-潮 G.Stork(2001) 366入 6色3 MR.Uskokovic et al/m Chem Soc190055 4
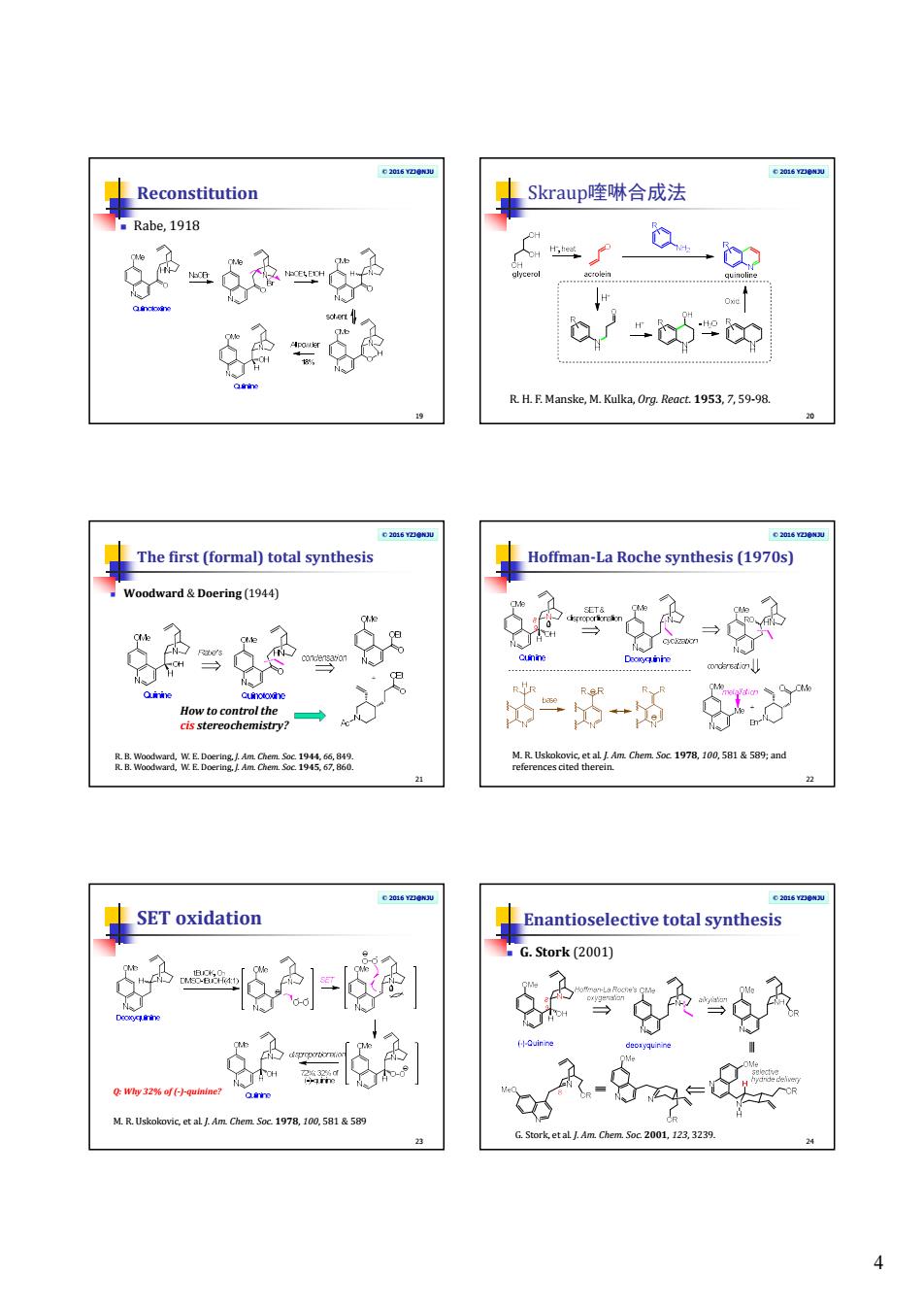
4 © 2016 YZJ@NJU Reconstitution Rabe, 1918 19 © 2016 YZJ@NJU Skraup喹啉合成法 20 R. H. F. Manske, M. Kulka, Org. React. 1953, 7, 59-98. © 2016 YZJ@NJU The first (formal) total synthesis Woodward & Doering (1944) 21 R. B. Woodward, W. E. Doering, J. Am. Chem. Soc. 1944, 66, 849. R. B. Woodward, W. E. Doering, J. Am. Chem. Soc. 1945, 67, 860. How to control the cis stereochemistry? © 2016 YZJ@NJU Hoffman-La Roche synthesis (1970s) 22 M. R. Uskokovic, et al. J. Am. Chem. Soc. 1978, 100, 581 & 589; and references cited therein. © 2016 YZJ@NJU SET oxidation 23 M. R. Uskokovic, et al. J. Am. Chem. Soc. 1978, 100, 581 & 589 Q: Why 32% of (-)-quinine? © 2016 YZJ@NJU Enantioselective total synthesis G. Stork (2001) 24 G. Stork, et al. J. Am. Chem. Soc. 2001, 123, 3239
Catalytic asymmetric synthesis(204) Organocatalysts →。 T Raheem,S.N Goodiman,E.N Jacobsen.f.Am Chem.Soc 2004,126,706. Organocatalyst 集影响世界历史的化合物 奎宁 Dig X-H;et al.Tetrahedron 2012.68,6240-6248. Taxol Semi-synthesis of Taxol 上客 皇7及型支皮的 5
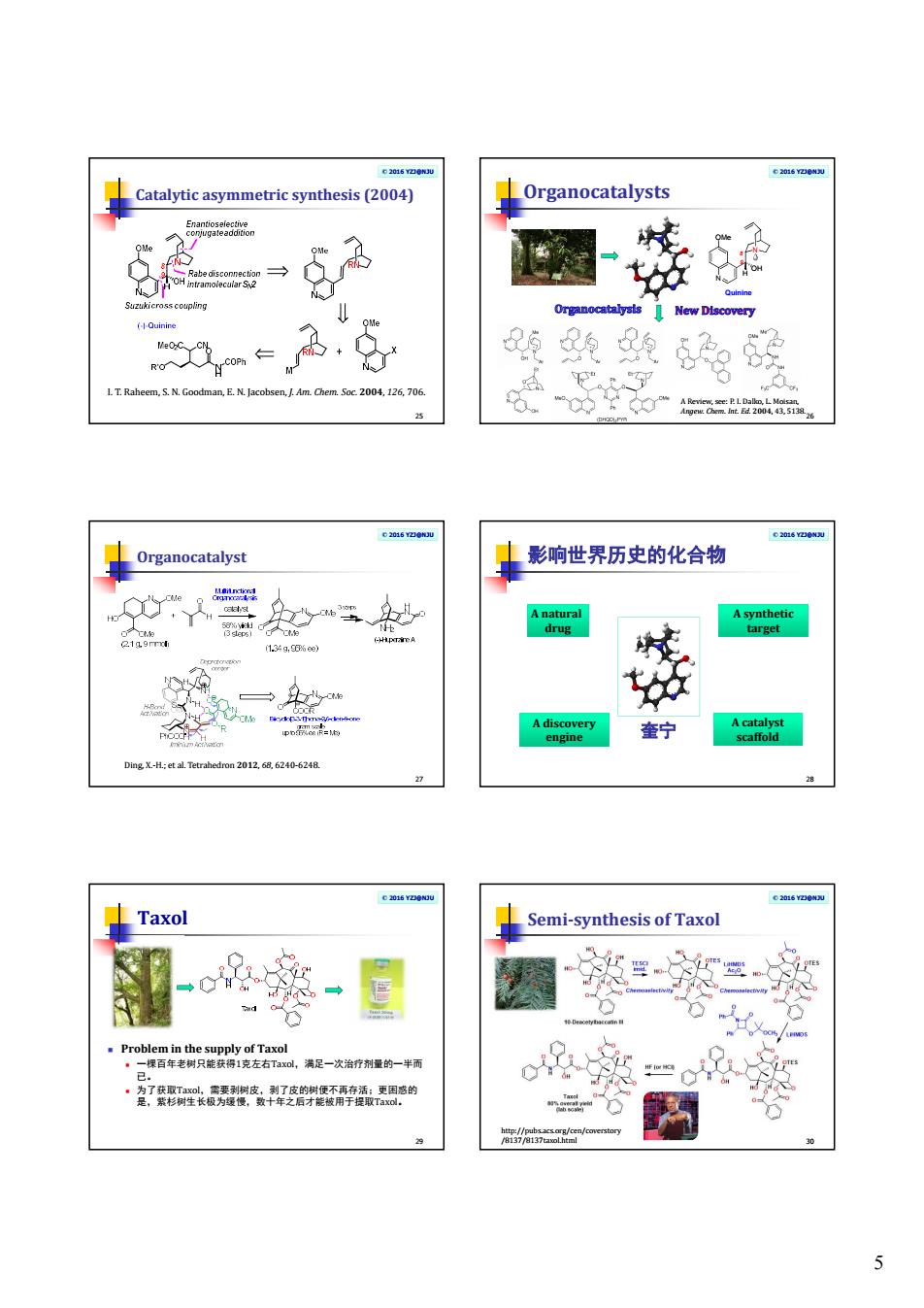
5 © 2016 YZJ@NJU Catalytic asymmetric synthesis (2004) 25 I. T. Raheem, S. N. Goodman, E. N. Jacobsen, J. Am. Chem. Soc. 2004, 126, 706. © 2016 YZJ@NJU Organocatalysts 26 N OMe OH H N Quinine 9 8 A Review, see: P. I. Dalko, L. Moisan, Angew. Chem. Int. Ed. 2004, 43, 5138. © 2016 YZJ@NJU Organocatalyst 27 Ding, X.-H.; et al. Tetrahedron 2012, 68, 6240-6248. © 2016 YZJ@NJU 影响世界历史的化合物 28 A natural drug A synthetic target A catalyst scaffold A discovery engine 奎宁 © 2016 YZJ@NJU Taxol Problem in the supply of Taxol 一棵百年老树只能获得1克左右Taxol,满足一次治疗剂量的一半而 已。 为了获取Taxol,需要剥树皮,剥了皮的树便不再存活;更困惑的 是,紫杉树生长极为缓慢,数十年之后才能被用于提取Taxol。 29 © 2016 YZJ@NJU Semi-synthesis of Taxol 30 http://pubs.acs.org/cen/coverstory /8137/8137taxol.html
Discovery of better drugs ET743 Camptothecin drugs 00 ↓Haaven((eribineyt间 19 gth thee are2 R By the tin ed by Ledfar,INeture 2010线6m (-)-Menthol _Asymmetric synthesis rmann&Reimer ()-menthol 1965 6
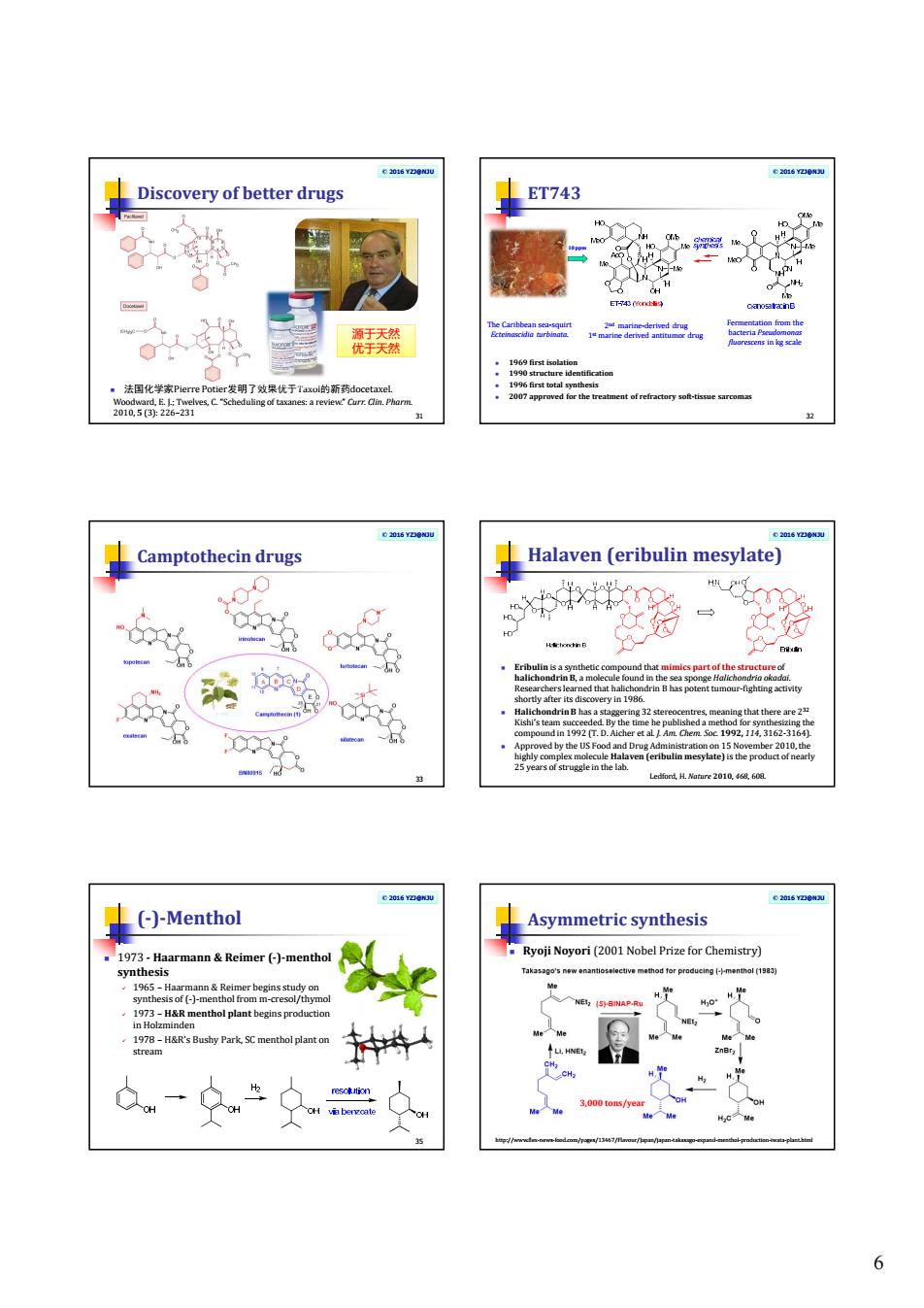
6 © 2016 YZJ@NJU Discovery of better drugs 法国化学家Pierre Potier发明了效果优于Taxol的新药docetaxel. 31 Woodward, E. J.; Twelves, C. “Scheduling of taxanes: a review.” Curr. Clin. Pharm. 2010, 5 (3): 226–231 源于天然 优于天然 © 2016 YZJ@NJU ET743 1969 first isolation 1990 structure identification 1996 first total synthesis 2007 approved for the treatment of refractory soft-tissue sarcomas 32 The Caribbean sea-squirt Ecteinascidia turbinata.10 ppm 2nd marine-derived drug 1 st marine derived antitumor drug Fermentation from the bacteria Pseudomonas fluorescens in kg scale © 2016 YZJ@NJU Camptothecin drugs 33 © 2016 YZJ@NJU Halaven (eribulin mesylate) Eribulin is a synthetic compound that mimics part of the structure of halichondrin B, a molecule found in the sea sponge Halichondria okadai. Researchers learned that halichondrin B has potent tumour-fighting activity shortly after its discovery in 1986. Halichondrin B has a staggering 32 stereocentres, meaning that there are 232 Kishi’s team succeeded. By the time he published a method for synthesizing the compound in 1992 (T. D. Aicher et al. J. Am. Chem. Soc. 1992, 114, 3162-3164). Approved by the US Food and Drug Administration on 15 November 2010, the highly complex molecule Halaven (eribulin mesylate) is the product of nearly 25 years of struggle in the lab. Ledford, H. Nature 2010, 468, 608. © 2016 YZJ@NJU (-)-Menthol 1973 - Haarmann & Reimer (-)-menthol synthesis 1965 – Haarmann & Reimer begins study on synthesis of (-)-menthol from m-cresol/thymol 1973 – H&R menthol plant begins production in Holzminden 1978 – H&R’s Bushy Park, SC menthol plant on stream 35 © 2016 YZJ@NJU Ryoji Noyori (2001 Nobel Prize for Chemistry) Asymmetric synthesis 3,000 tons/year http://www.flex-news-food.com/pages/13467/Flavour/Japan/japan-takasago-expand-menthol-production-iwata-plant.html

上化学分子与生态 Flavoring Materials 59cpioeyofmethy1dhydroasmomate Juvenlle Hormone 6级14-1552000 Historical footprint(1828-1944) Woodward-Corey时代(1945-1990) 金益品高 27 n2 m121g The Woodward"Hallmark" Frontier molecular orbital theory VB,2 synthesis and Woodward-Hoffmann rules 构康重通和立体选娜性拉碱 12131425
7 © 2016 YZJ@NJU 化学分子与生态 Insect pheromone (Communication) 37 Archives of Insect Biochemistry and Physiology 68:144–155 (2008) The structures of the different juvenile hormones © 2016 YZJ@NJU Flavoring Materials 1959 – Discovery of methyl dihydrojasmonate (Hedione®) 1971 – Introduction of Hedione® 1996 - First synthesis of pure (+)-cis-Hedione® "the olfactively most active stereoisomer of Hedione®” 38 O O O © 2016 YZJ@NJU Historical footprint (1828-1944) 39 © 2016 YZJ@NJU Woodward-Corey 时代 (1945-1990) 40 Co © 2016 YZJ@NJU The Woodward “Hallmark” 41 © 2016 YZJ@NJU Frontier molecular orbital theory VB12 synthesis and Woodward-Hoffmann rules 42 . Woodward formulated his ideas (which were based on the symmetry properties of molecular orbitals) based on his experiences as a synthetic organic chemist; he asked Hoffman to perform theoretical calculations to verify these ideas, which were done using Hoffmann's Extended Hückel method. The predictions of these rules, called the "Woodward-Hoffmann rules" were verified by many experiments. Hoffmann shared the 1981 Nobel Prize for this work along with Kenichi Fukui, a Japanese chemist who had done similar work using a different approach; Woodward undoubtedly would have received a second Nobel Prize as well had he lived. . (see: http://en.wikipedia.org/wiki/Robert_Burns_Woodward) Hoffmann, R.; Woodward, R. B. (1970). "Orbital Symmetry Control of Chemical Reactions". Science 167 (3919): 825–831. 1970 Feb 6. doi:10.1126/science.167.3919.825

E.J.Corey's Retrosynthesis 世纪之交10902010) 海葵毒素(Palytoxin) A red tide o Famous syntheses(1995-2010) Phorboxazoles 8
8 © 2016 YZJ@NJU E. J. Corey’s Retrosynthesis In 1990, E. J. Corey won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. The Logic of Chemical Synthesis 43 E. J. Corey, “Retrosynthetic Thinking - Essentials and Examples”. Chem. Soc. Rev. 1988, 17: 111–133. E. J. Corey, X-M. Cheng (1995). The Logic of Chemical Synthesis. New York: Wiley. E. J. Corey, "The Logic of Chemical Synthesis: Multistep Synthesis of Complex Carbogenic Molecules (Nobel Lecture)“ Angew. Chem. Int. Ed. Engl. 1991, 30 (5): 455–465. © 2016 YZJ@NJU 世纪之交(1990-2010) 44 K. C. Nicolaou © 2016 YZJ@NJU 海葵毒素(Palytoxin) 45 Palytoxin is a very dangerous toxin; it is considered to be one of the most toxic non-peptide substances known, second only to maitotoxin in terms of toxicity in mice. Palytoxin is a natural compound that is produced by several marine species and can be found in many more species due to accumulation. Palytoxin was originally isolated in 1971 in Hawaii from the seaweed-like coral “Limu make o hana (Seaweed of Death from Hana)”. Later, in 1982 its full chemical structure was published by Prof. Daisuke Uemura and co-workers at Nagoya University. Professor Yoshito Kishi's group at Harvard University first synthesized palytoxin in 1994. R.W. Armstrong, J.-M. Beau, S.H. Cheon, W.J. Christ, H. Fujioka, W.-H. Ham, L.D. Hawkins, H. Jin, S.H. Kang, Y. Kishi, M.J. Martinelli, W.W. McWhorter, Jr., M. Mizuno, M. Nakata, A.E. Stutz, F.X. Talamas, M. Taniguchi, J.A. Tino, K. Ueda, J. Uenishi, J.B. White, and M. Yonaga, "Total Synthesis of Palytoxin Carboxylic Acid and Palytoxin Amide". J. Am. Chem. Soc. 1989, 111: 7530. Suh EM and Kishi Y. "Synthesis of Palytoxin from Palytoxin Carboxylic Acid". J. Am. Chem. Soc. 1994,116: 11205. © 2016 YZJ@NJU Marine polyether toxins 日本天然产物界的雄厚积累 46 A red tide © 2016 YZJ@NJU Famous syntheses (1995-2010) 47 © 2016 YZJ@NJU Phorboxazoles 48 Isolated via methanolic extract of the marine sponge Phorbas sp. from the coast of Muiron Island, Western Australia . Searle, P. A.; Molinski, T. F. J. Am. Chem. Soc. 1995, 117, 8126; Searle, P. A.; Molinski, T. F. J. Am. Chem. Soc. 1996, 118, 9422
_Ecteinascidin 743 Selected syntheses(2000-2012) Selected syntheses(2000-2012) Selected syntheses(000-1) 叠 器 Selectedsyntheses(2000-2012) Selected syntheses(2000-2012) 。 9
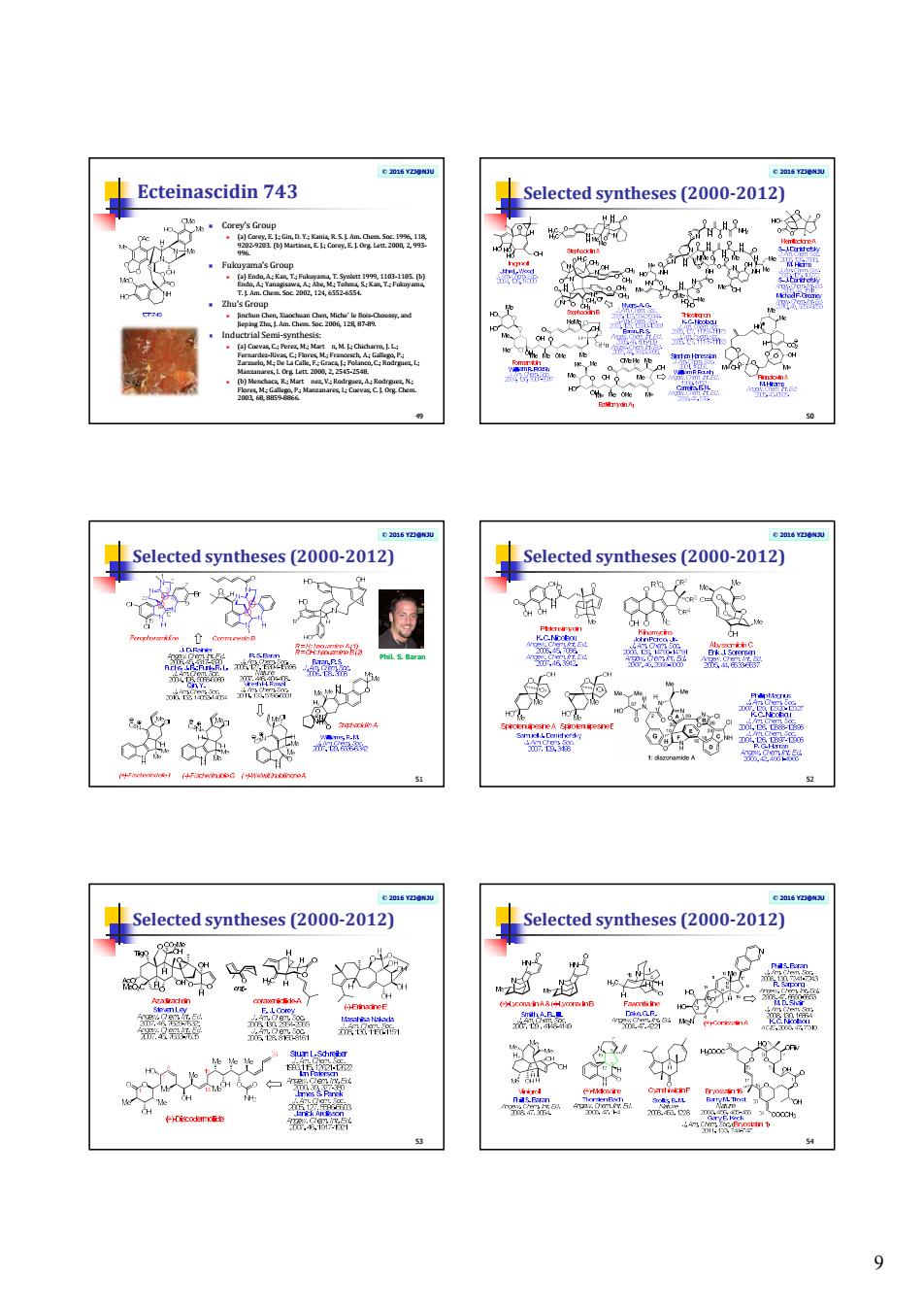
9 © 2016 YZJ@NJU Ecteinascidin 743 Corey's Group (a) Corey, E. J.; Gin, D. Y.; Kania, R. S. J. Am. Chem. Soc. 1996, 118, 9202-9203. (b) Martinez, E. J.; Corey, E. J. Org. Lett. 2000, 2, 993- 996. Fukuyama's Group (a) Endo, A.; Kan, T.; Fukuyama, T. Synlett 1999, 1103-1105. (b) Endo, A.; Yanagisawa, A.; Abe, M.; Tohma, S.; Kan, T.; Fukuyama, T. J. Am. Chem. Soc. 2002, 124, 6552-6554. Zhu's Group Jinchun Chen, Xiaochuan Chen, Miche` le Bois-Choussy, and Jieping Zhu, J. Am. Chem. Soc. 2006, 128, 87-89. Inductrial Semi-synthesis: (a) Cuevas, C.; Perez, M.; Mart n, M. J.; Chicharro, J. L.; Fernardez-Rivas, C.; Flores, M.; Francesch, A.; Gallego, P.; Zarzuelo, M.; De La Calle, F.; Graca, J.; Polanco, C.; Rodrguez, I.; Manzanares, I. Org. Lett. 2000, 2, 2545-2548. (b) Menchaca, R.; Mart nez, V.; Rodrguez, A.; Rodrguez, N.; Flores, M.; Gallego, P.; Manzanares, I.; Cuevas, C. J. Org. Chem. 2003, 68, 8859-8866. 49 © 2016 YZJ@NJU Selected syntheses (2000-2012) 50 © 2016 YZJ@NJU Selected syntheses (2000-2012) 51 Phil. S. Baran © 2016 YZJ@NJU Selected syntheses (2000-2012) 52 © 2016 YZJ@NJU Selected syntheses (2000-2012) 53 © 2016 YZJ@NJU Selected syntheses (2000-2012) 54

Selectedsyntheses(2000-2012) Selected syntheses(2000-2012) _Selected syntheses(2000-2012) ↓elededy2oo2o1 学 The way we've traveled. Organic synthesis,where now? “艺术”境界 。精淮 。高效 近乎无人之境 ·预有员角装纳然包括天然的、人灯 10
10 © 2016 YZJ@NJU Selected syntheses (2000-2012) 55 © 2016 YZJ@NJU Selected syntheses (2000-2012) 56 © 2016 YZJ@NJU Selected syntheses (2000-2012) 57 © 2016 YZJ@NJU Selected syntheses (2000-2012) 58 © 2016 YZJ@NJU The way we’ve traveled. “艺术”境界 精准 高效 经济 实用 近乎无人之境 所有具有合理结构的分子,包括天然的、人工 设计的、和理论推测的分子。 59 © 2016 YZJ@NJU Organic synthesis, where now? 60 Dieter Seebach is a German chemist known for his synthesis of biopolymers and dendrimers, and for his contributions to stereochemistry. He was born on 31 October 1937 in Karlsruhe. He studied chemistry at the University of Karlsruhe (TH) under the supervision of Rudolf Criegee and at Harvard University with Elias Corey finishing in 1969. After his habilitation he became professor for organic chemistry at the University of Giessen. After six years he was appointed professor at the ETH Zurich where he worked until he retired in 2003