
南 现代有机合成化学 复杂分子合成设与全台成同 Strychnine(的士宁,番木置碱马钱子碱: 姚祝军 南京大学化学化工学院/微结构国家实验室 Email:yaoz@nju.edu.cn 趣两 2016年6月 Strychnine Biological properties 8腰 schnne5apowerflnewrototawhosemodeod dote for s 09.5630330 Landmarks 集Biosynthesis ≤- a prove y woeaoanadtesiaamhessod
1 姚祝军 南京大学化学化工学院/微结构国家实验室 Email: yaoz@nju.edu.cn 2016年6月 1 现代有机合成化学 复杂分子合成设计与全合成 (8) 南京大学化学化工学院 2015级研究生课程 Strychnine (的士宁, 番木鳖碱, 马钱子碱): An excellent platform for organic synthesis © 2016 YZJ@NJU Strychnine Strychnine was the first alkaloid to be identified in plants of the genus Strychnos, family Loganiaceae. Strychnos, named by Carl Linnaeus in 1753, is a genus of trees and climbing shrubs of the gentian order. Strychnine is a highly toxic (LD50 = 0.16 mg/kg in rats, 1–2 mg/kg orally in humans), colorless crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine causes muscular convulsions and eventually death through asphyxia. The most common source is from the seeds of the Strychnos nux vomica tree. 3 http://en.wikipedia.org/wiki/Strychnine © 2016 YZJ@NJU Biological properties Strychnine is a powerful neurotoxin whose mode of action is well characterized. The alkaloid competitively binds to the glycine receptor chloride channel (also named the strychninesensitive glycine receptor, Kd in the nanomolar range), eliminating glycinergic inhibition in the spinal cord. There is no direct antidote for strychnine poisoning, which is treated by administering anticonvulsants and muscle relaxants to ease the convulsions. 4 A recent review: J. W. Lynch, Neuropharmacology2009, 56, 303-309. © 2016 YZJ@NJU Landmarks One of the first to be isolated and purified, yet one of the last to have its structure established using largely classical methods. Characterizing the structure of strychnine were pursued throughout the early 20th century, led by Leuchs and Robinson et al. who published nearly 400 papers addressing strychnines structure using degradative methods. Finally, Woodward and Brehm proved the correct structure in 1948 from a combination of degradative evidence and a key use of UV spectroscopy. The connectivity and absolute configuration of strychnine were soon confirmed by X-ray crystallography. Woodward et al. announced the first total synthesis of strychnine in 1954. 5 © 2016 YZJ@NJU Biosynthesis Nearly 30 naturally occurring Strychnos alkaloids are now recognized. As with all monoterpene indole alkaloids, their biosynthesis originates from the union of tryptamine and secologanin. 6 N. G. Bisset, in Indole and Biogenetically Related Alkaloids (Eds.: J. D. Phillipson, M. H. Zenk), Academic Press, London, 1980, pp. 27 – 61

陶 Total and formal syntheses 舞 满 先82微8- Woodwards tandmarkymtheas ( Woodward synthesis(1) l9d世 05 Use of markedprecursor of a reactive unit Woodward synthesis2(☒ Overman's Synthesis (1993) 子 ¥8 其等竖 2
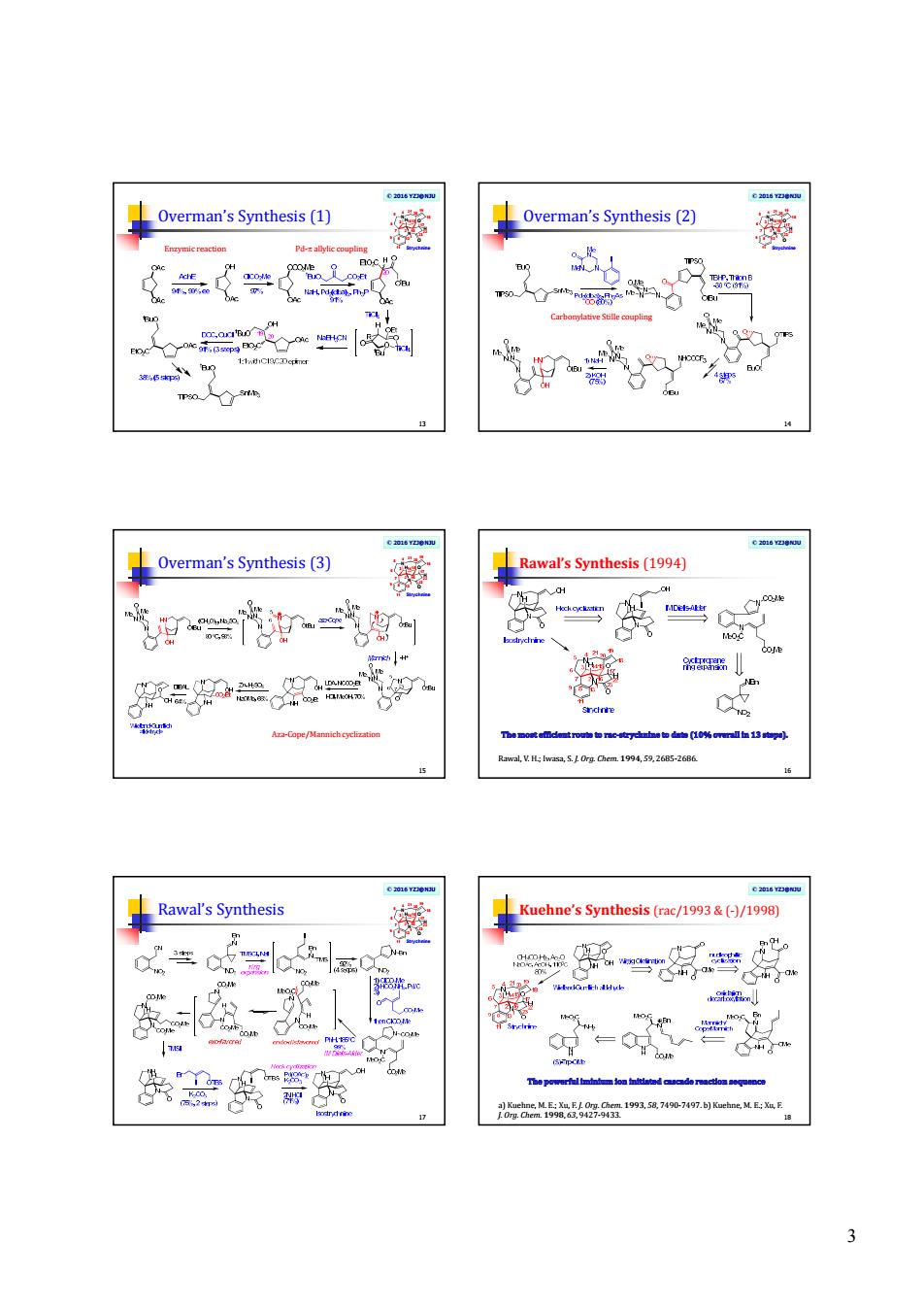
2 Total synthesis of strychnine Advancing synthetic methodologies 7 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 A recent review, see: Jeffrey S. Cannon and Larry E. Overman*, Angew. Chem., Int. Ed. (2012), DOI: 10.1002/anie.201107385. © 2016 YZJ@NJU Total and formal syntheses To 2011, 17 total and formal syntheses of strychnine have been reported. All syntheses of strychnine have intercepted one of two common intermediates: isostrychnine or the Wieland–Gumlich aldehyde. 8 M. E. Kuehne, F. Xu, J. Org. Chem. 1993, 58, 7490 – 7497. F. A. L. Anet, R. Robinson, Chem. Ind. 1953, 245. © 2016 YZJ@NJU Woodward’s Landmark Synthesis (1954) 9 a) R. B. Woodward, M. P. Cava, W. D. Ollis, A. Hunger, H. U. Daeniker, K. Schenker, J. Am. Chem. Soc. 1954, 76, 4749 – 4751; b) R. B. Woodward, M. P. Cava, W. D. Ollis, A. Hunger, H. U. Daeniker, K. Schenker, Tetrahedron1963, 19, 247 – 288. © 2016 YZJ@NJU Woodward synthesis (1) 10 • Use of marked precursor of a reactive unit © 2016 YZJ@NJU Woodward synthesis (2) 11 • Intramolecular delivery of a reagent © 2016 YZJ@NJU Overman’s Synthesis (1993) 12 a) Knight, S. D.; Overman, L. E.; Pairaudeau, G. J. Am. Chem. Soc. 1993, 115, 9293 – 9294; b) Knight, S. D.; Overman, L. E.; Pairaudeau, G. J. Am. Chem. Soc. 1995, 117, 5776 – 5788
Overman's Sythesis( 0 verma's Synthesis 。= Overman's Synthesis (3) 薆 Rawal's Synthesis(1994) Q61 Rawal's Synthesis Kuehne's Synthesis (c) 盐需函
3 © 2016 YZJ@NJU Overman’s Synthesis (1) 13 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 Enzymic reaction Pd-p allylic coupling © 2016 YZJ@NJU Overman’s Synthesis (2) 14 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 Carbonylative Stille coupling © 2016 YZJ@NJU Overman’s Synthesis (3) 15 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 Aza-Cope/Mannich cyclization © 2016 YZJ@NJU Rawal’s Synthesis (1994) 16 Rawal, V. H.; Iwasa, S. J. Org. Chem. 1994, 59, 2685-2686. © 2016 YZJ@NJU Rawal’s Synthesis 17 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 © 2016 YZJ@NJU Kuehne’s Synthesis (rac/1993 & (-)/1998) 18 a) Kuehne, M. E.; Xu, F. J. Org. Chem. 1993, 58, 7490-7497. b) Kuehne, M. E.; Xu, F. J. Org. Chem. 1998, 63, 9427-9433

Kuehne's Synthesis(1) 薆 Kuehne's Synthesis( 楚 澄澄 混"↓ 世兰料 尝”问学阶新 p 常禁益条琴离 1: 篱 Martin's Synthesis (2001) 集Martin's Synthesis 簧 格m
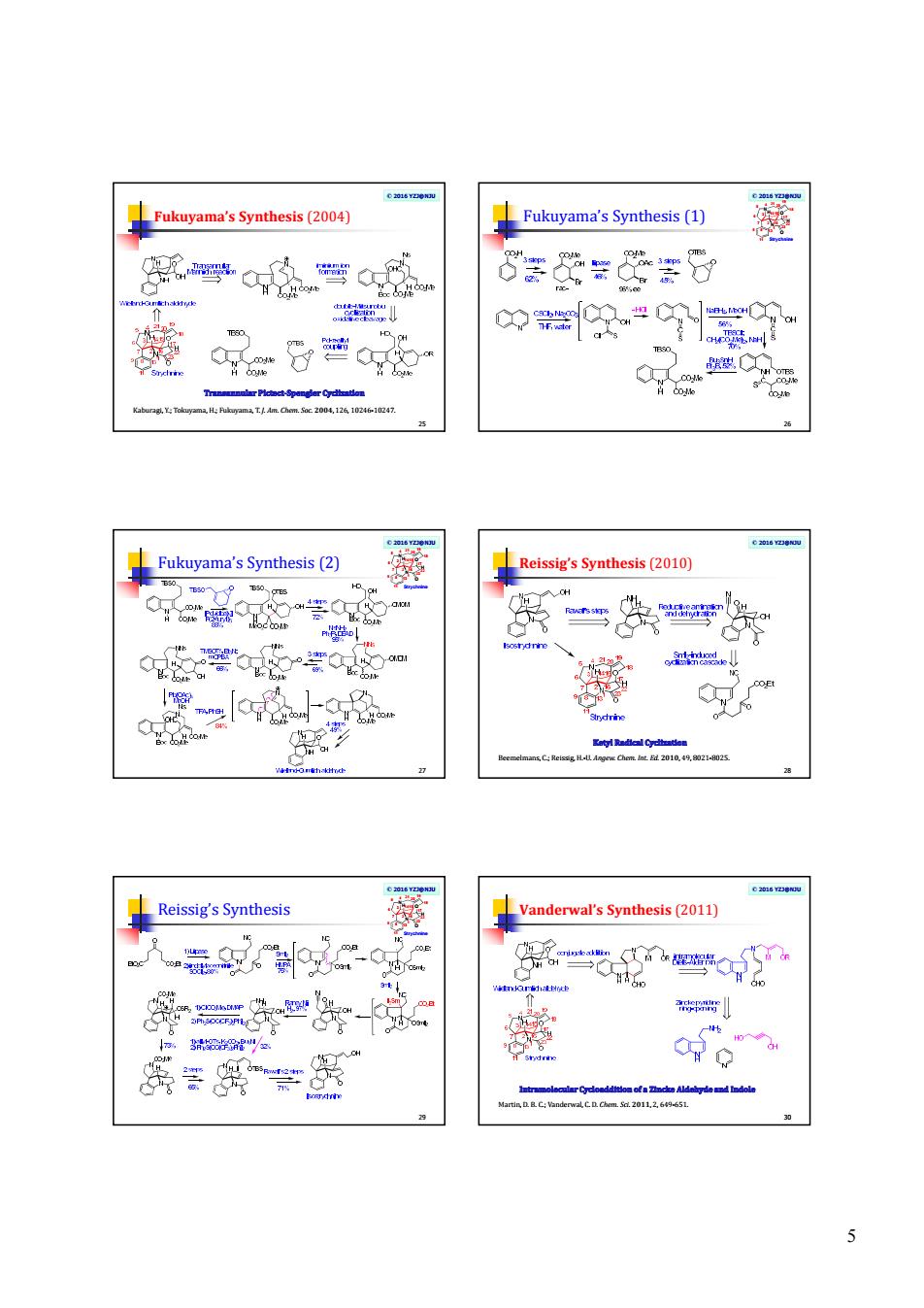
4 © 2016 YZJ@NJU Kuehne’s Synthesis (1) 19 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 © 2016 YZJ@NJU Kuehne’s Synthesis (2) 20 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 © 2016 YZJ@NJU Vollhardt’s Synthesis (2000) 21 Eichberg, M. J.; Dorta, R. L.; Lamottke, K.; Vollhardt, K. P. C. Org. Lett. 2000, 2, 2479- 2481. © 2016 YZJ@NJU Vollhardt’s Synthesis 22 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 © 2016 YZJ@NJU Martin’s Synthesis (2001) 23 a) Martin, S. F.; Clark, C. W.; Ito, M.; Mortimore, M. J. Am. Chem. Soc. 1996, 118, 9804-9805; b) Ito, M.; Clark, C. W.; Mortimore, M.; Martin, S. F. J. Am. Chem. Soc. 2001, 123, 8003-8010. © 2016 YZJ@NJU Martin’s Synthesis 24 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23
Fakuyama's Synthesis(2o0 Synthesis (1 8器安安尝8 ∞头2离 Fukuyama's Synthesis (2) Reissig's Syathesis (2010) 0或“等8 Ooo a黑↓ 8我 Reissig's Synthesis Vanderwal's Synthesis(2011) 公 多器公 奔=墨
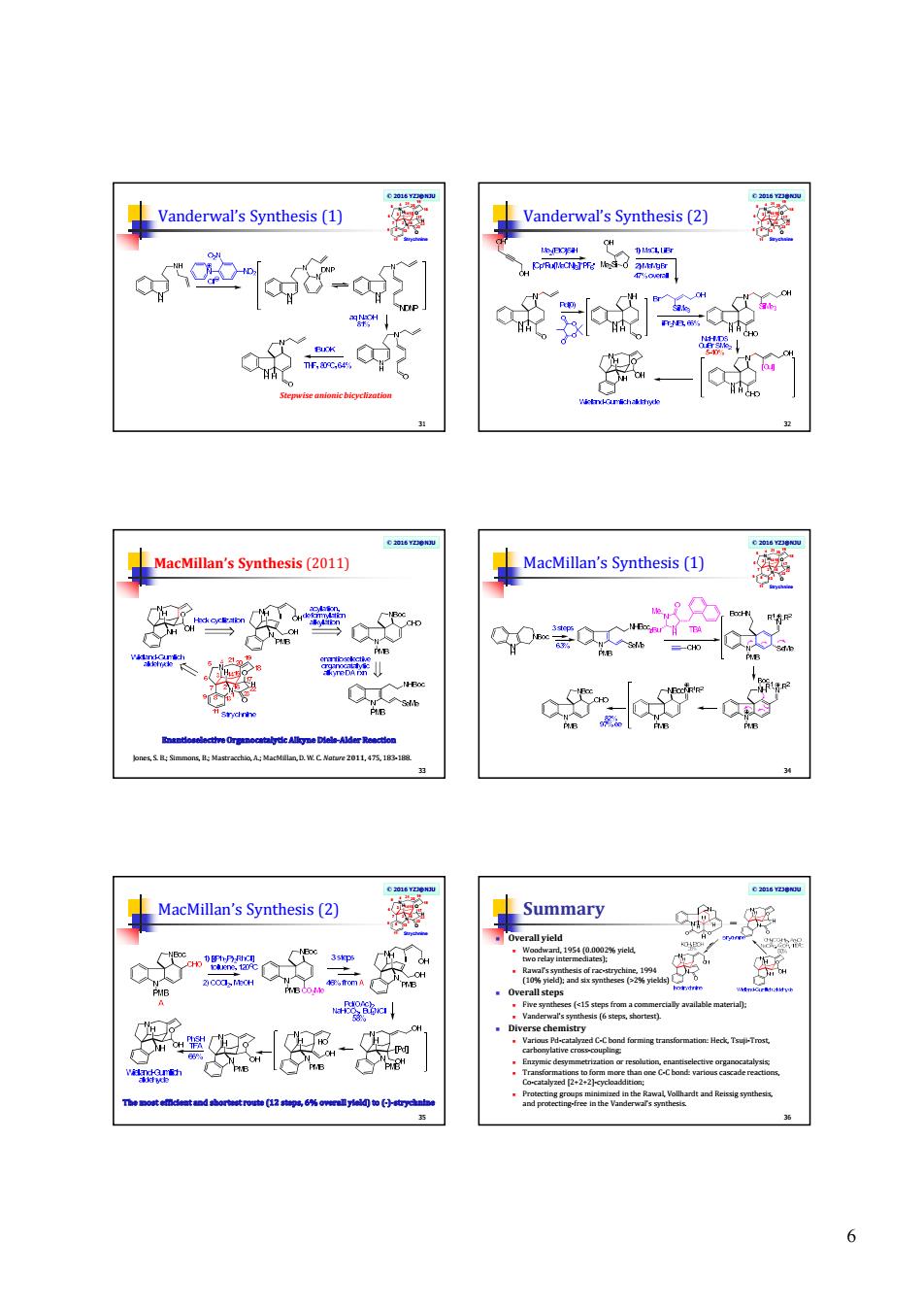
5 © 2016 YZJ@NJU Fukuyama’s Synthesis (2004) 25 Kaburagi, Y.; Tokuyama, H.; Fukuyama, T. J. Am. Chem. Soc. 2004, 126, 10246-10247. © 2016 YZJ@NJU Fukuyama’s Synthesis (1) 26 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 © 2016 YZJ@NJU Fukuyama’s Synthesis (2) 27 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 © 2016 YZJ@NJU Reissig’s Synthesis (2010) 28 Beemelmans, C.; Reissig, H.-U. Angew. Chem. Int. Ed. 2010, 49, 8021-8025. © 2016 YZJ@NJU Reissig’s Synthesis 29 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 © 2016 YZJ@NJU Vanderwal’s Synthesis (2011) 30 Martin, D. B. C.; Vanderwal, C. D. Chem. Sci. 2011, 2, 649-651
Vanderwal's Synthesis(1) 菱 人 "↓ 益的 战系是 /7 严↓ 器 oSzow.og MacMllan's Synthesis (2) 魔 Summary c5三 6的a 禁-殿 6
6 © 2016 YZJ@NJU Vanderwal’s Synthesis (1) 31 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 Stepwise anionic bicyclization © 2016 YZJ@NJU Vanderwal’s Synthesis (2) 32 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 © 2016 YZJ@NJU MacMillan’s Synthesis (2011) 33 Jones, S. B.; Simmons, B.; Mastracchio, A.; MacMillan, D. W. C. Nature 2011, 475, 183-188. © 2016 YZJ@NJU MacMillan’s Synthesis (1) 34 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 © 2016 YZJ@NJU MacMillan’s Synthesis (2) 35 N N O O H H Strychnine 1 2 3 7 4 5 6 9 8 11 13 1415 16 17 18 19 20 21 22 23 © 2016 YZJ@NJU Summary Overall yield Woodward, 1954 (0.0002% yield, two relay intermediates); Rawal's synthesis of rac-strychine, 1994 (10% yield); and six syntheses (>2% yields) Overall steps Five syntheses (<15 steps from a commercially available material); Vanderwal's synthesis (6 steps, shortest). Diverse chemistry Various Pd-catalyzed C-C bond forming transformation: Heck, Tsuji-Trost, carbonylative cross-coupling; Enzymic desymmetrization or resolution, enantiselective organocatalysis; Transformations to form more than one C-C bond: various cascade reactions, Co-catalyzed [2+2+2]-cycloaddition; Protecting groups minimized in the Rawal, Vollhardt and Reissig synthesis, and protecting-free in the Vanderwal's synthesis. 36